V. Stathopoulou1, I. Liapis2
1DVM, GPCert SAM, GPCertEndo (ESVPS), PgCertSAM (Harper Adams University, UK)
2DVM, Cert. Ophthalmology
Keywords:
amoxicillin/clavulanic acid, cat, doxycycline, clindamycin, oesophageal stricture
Abstract
Drug-induced oesophagitis (DO) resulting in benign oesophageal strictures (BOS) is a pathological condition with limited reports in cats, dogs and human in the literature. In this case series, three feline cases of oesophageal stricture subsequent to doxycycline, clindamycin and amoxicillin/clavulanic acid administration are reported. In all cases, the predominant clinical signs included salivation, regurgitation of dry food and weight loss with normal appetite. Diagnosis was reached by standard or contrast-enhanced radiographic evaluation and confirmed by oesophagoscopy, during which typical oesophagitis lesions were absent. The stricture site in all three cases was located in the cranial intrathoracic oesophagus. Balloon dilation under endoscopic guidance was undertaken in order to resolve the BOS with a favourable outcome in two cats after 12 and 3 balloon dilation sessions respectively, performed at 3 to7-day intervals, whereas, in the third cat the severity of the stricture resulted in dilation failure and oesophageal perforation. The two cats remained asymptomatic for 4 years and 14 months respectively, while the third case was euthanised at the owner’s request. The present case series includes the first report of oesophageal stricture subsequent to amoxicillin/clavulanic acid administration and underlines the need for food or water consumption to follow any oral administration of medicines to cats. In addition, it is demonstrated that the number of balloon dilation sessions necessary to resolve the strictures may exceed what is commonly reported in the literature.
Introduction
Acquired benign oesophageal strictures (BOS) are uncommon in dogs and cats.1,2 The main potential cause is severe oesophagitis, in which inflammation is not confined to the oesophageal mucosa, but extends to the submucosal and muscular layer of the oesophagus, resulting in intramural fibrosis.3 Oesophagitis in cats is usually the result of gastroesophageal/duodenogastric reflux (GDOR) secondary to general anaesthesia, especially in operations requiring manipulation of intra-abdominal structures. It may also occur due to the presence of foreign bodies (including hairballs), ingestion of irritants/corrosive chemical substances, systemic disorders resulting in chronic vomiting, oral medications, mainly in the form of capsules that were not accompanied by food or water swallow, hiatal hernia, and rarely feline calicivirus infection.4,5 Erosions of the oesophageal mucosal lesions and subsequent stricture formation due to oral tablets and capsules have been reported in both cats and people.6,7 Medications that have been implicated for oesophagitis and oesophageal strictures in cats include tetracyclines (in particular doxycycline) and clindamycin, in the form of tablets or capsules administered without being accompanied by a food or water swallow.8-12 Clinical signs of oesophageal strictures manifest 3-16 days following the onset of treatment, and the usual fibrous ring formation sites are located in the middle segment of the cervical oesophagus and in the thoracic oesophagus, at the level of the heart base.4,5,9 The severity of clinical signs depends on the site and size of the stricture and clinical signs include regurgitation of mainly solid foods, salivation, and weight loss with normal appetite.13-15 In cases of aspiration pneumonia, due to secondary oesophageal dilation cranial to the stricture site, depression, fever and respiratory signs are noted.16 Diagnosis is guided by barium oesophagography, fluoroscopy and oesophagoscopy, and treatment intervention techniques include stricture dilation via oesophageal bougienage or endoscopy-guided balloon catheters, or even the tip of the endoscope under direct endoscopic visualisation, or the use of metal or biodegradable self-expanding stents and surgical reconstruction.11,16-18 The present study describes three feline cases of BOS following administration of doxycycline, clindamycin and amoxicillin/clavulanic acid, aiming to highlight the risk of oral medication administration not accompanied by food or water swallow (dry swallow) to cats, to add the combination of amoxicillin/clavulanic acid to the antimicrobials implicated for stricture formation, and to discuss the number of balloon dilation sessions necessary for stricture management.
Interesting case 1 (IC1)
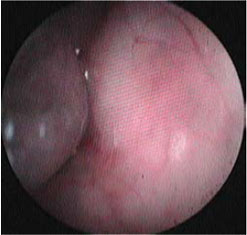
A six-month-old female intact domestic short-haired cat, weighing 2.2 kg, was admitted due to severe weight loss noted throughout the previous month, dysphagia and regurgitation of dry food. Appetite was normal and the cat was able to consume small amounts of liquefied food. The cat had an indoor /outdoor lifestyle, lived with two other cats and was fully vaccinated. According to the history, 16 days prior to the onset of regurgitations, it was diagnosed with Mycoplasma haemofelis infection and received oral prednisolone (Prezolon, Takeda Hellas SA) at a dose of 1 mg kg-1 twice daily (BID) and doxycycline (Ronaxan 20, Merial) at a dose of 10 mg kg-1 BID for a duration of two weeks. The tablets were administered without food or water. Physical examination revealed poor body condition with a body condition score of 3/9 (ideal body weight corresponding to 5/9), 8% dehydration with dry mucous membranes, a decrease in the skin turgor test and a slightly increased capillary refill time. Mucous membranes were pale. A complete blood count revealed regenerative (absolute reticulocyte count of 140,000/μL) anaemia (haematocrit 24.5%, reference range 30-45%), mild leucocytosis (19.4 x 109/L, reference range 5.5-16.9 x 109/L) and neutrophilia (16.8 x 109/L; reference range 2.5-12.5 x 109/L). No haemoparasites were detected in the peripheral blood smear. Serum biochemistry results were within the normal reference range, whereas serology testing for feline leukaemia virus and feline immunodeficiency virus was negative (Snap FIV/FelV, Idexx). Lateral radiographs obtained post barium sulfate meal revealed localised narrowing of the oesophageal lumen and dilation cranial to the stricture site. (Figure 1). Oesophagoscopy was performed with a flexible video endoscope (Pentax EG-1690K, 5.4 mm flexible portion diameter, 2.00 mm forceps channel diameter, 1,010 mm working length). No lesions were found on the oesophageal mucosa, the stricture was detected in the cranial thoracic segment, and it was 2.6-3 mm in diameter (Figure 2). Estimation of the diameter of the oesophageal lumen at the stricture site was obtained by Foley catheters. Foley 8 Fr (1 Fr =0.33mm) catheter passage through the stricture site was possible, yet the same could not be accomplished with the distal end of the endoscope (5.4 mm). In order to dilate the stricture, an endoscopic wire-guided dilation balloon that can produce three distinct diameters was used (CRE PRO wire guided 6-7-8 mm, length 5.5 cm, catheter size 7.5 Fr, Boston scientific). Bleeding was limited at the procedure site, and the procedure was repeated for 12 sessions in total, with a 3-5-day interval between sessions, over a 45-day period due to recurrence of the stricture and clinical signs. Medical treatment post-dilation included the administration of a low-fat canned diet, prednisolone (Prezolon inj. sol 25 mg/1 ml, Takeda Hellas SA) [1 mg kg-1, SID, subcutaneously (SC), for 1 month] amoxicillin/clavulanic acid (Synulox RTU, Pfizer) [15 mg kg-1, once a day, (SID), SC, for 2 weeks], sucralfate (Peptonorm oral susp. 1000 mg/5 ml, Uni-Pharma SA) [75 mg, twice a day (BID), per os, for 1 month] and tramadol (Tramal inj.sol 100 ml/2 ml amp, Vianex SA) (2 mg kg-11, BID, SC, for the first 2 days after each session). After the final session, the cat could receive both canned and dry food and recovery, physical growth and development was uneventful. Four years later, the cat remains asymptomatic.
 |
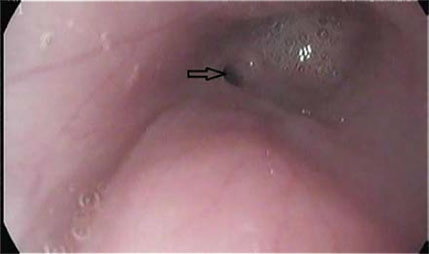 |
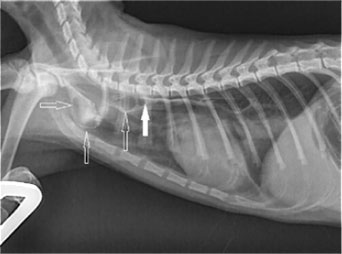
| Figure 1: Interesting case 1. Lateral thoracic radiograph after administration of barium in a liquefied meal. The dilation of the cranial thoracic oesophagus (open arrows) is shown, cranial to the stricture site (block arrow). | Figure 2: Interesting case 1. Endoscopic appearance of the oesophageal stricture (arrow). Note the lumen dilation cranial to the stricture. |
Interesting case 2 (IC2)
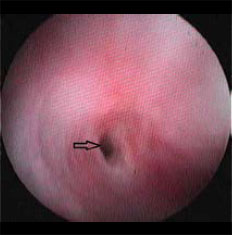 |
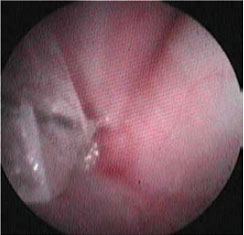
| Figure 3: Interesting case 2. Endoscopic appearance of the oesophageal stricture (arrow). Note the mucosal hyperaemia due attempting to insert the distal end of the endoscope through the stricture. |
A male intact, 3-year-old, domestic short-haired cat, weighing 5.1 kg, was admitted for chronic vomiting and regurgitation, mainly of dry food, for 2 weeks, and weight loss with normal appetite. According to the history, 30 days prior to admission, fever (40.2 0C), depression and a mature right facial subcutaneous abscess from a fight wound had been noted. The abscess had been drained, lavage had been performed with chlorhexidine solution and clindamycin (Antirobe Caps 75 mg, Pfizer) had been orally administered (11 mg kg-1, BID, per os for 2 weeks). For the abscess lancing and drainage procedure, induction was accomplished by isoflurane in an anaesthetic/oxygen cage and general anaesthesia was maintained via mask, and the whole procedure lasted 15 minutes. Physical examination three weeks post-surgery revealed a moderate body condition, with a body condition score of 4/9 and 7% dehydration. In order to evaluate appetite, fresh canned food was offered and the cat attempted to consume it but showed signs of dysphagia, salivation, regurgitation and coughing. The results of both complete blood count and biochemistry were within normal range. Survey lateral thoracic radiograph was unremarkable. The suspected oesophageal stricture was confirmed by oesophagoscopy with a flexible fiberopticendoscope (Pentax FG-24V, 7.9 mm distal end diameter, 2.4 mm forceps channel diameter, 1,050 mm working length). No lesions were observed on the oesophageal mucosa, and the stricture was detected in the cranial thoracic segment, at the level of the heart base (Figure 3), with an estimated size of 3.5-4 mm, considering that it was possible for a Foley 12 Fr catheter to pass through. An endoscopic wire-guided dilation balloon (Mediglobe, 12 mm, 8 cm, 70 PSI/5 Atm) was used to dilate the stricture (Figures 4-6). There was limited bleeding at the procedure site, and the procedure was repeated for 3 sessions in total, with weekly intervals between sessions, due to recurrence of stricture over a 21-day period (Figure 7). Post dilation, medical treatment was the same as that of Case 1. After the final session and 14 months later, no relapse was reported, and the cat continues to be fed mixed canned and dry food.
 |
 |
 |
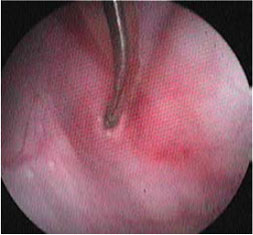
| Figure 4: Interesting case 2. Stricture dilation by endoscopic dilation balloon. First stage: Introducing the non-inflatable end of the balloon into the stricture site. | Figure 5: Interesting case 2. Stricture dilation by endoscopic dilation balloon. Second stage: Introducing the inflatable part of the balloon into the stricture site. | Figure 6: Interesting case 2. Stricture dilation by endoscopic dilation balloon. Third stage: filling the inflatable part of the balloon and dilating the stricture. |
 |
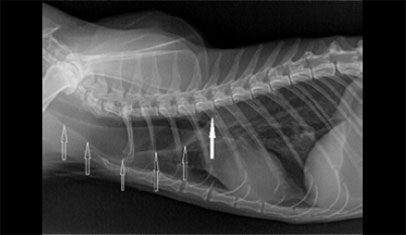 |
| Figure 7: Interesting case 2. Endoscopic appearance of oesophageal stricture site immediately after dilation by an endoscopic dilation balloon. The arrows mark the edges of the traumatic rupture of the fibrous ring of the stricture stenosis. The procedure is accompanied by limited bleeding. | Figure 8: Interesting case 3. Lateral thoracic radiograph. The dilation of the cranial thoracic oesophagus is shown (open arrows), cranial to the stricture site (block arrow). |
Interesting case 3 (IC3)
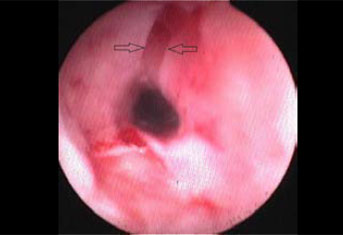
A female spayed, 13-year-old Persian cat, weighing 2.1 kg, was admitted with a history of regurgitation of both dry and canned food, of 4 weeks duration. Five weeks prior to admission, it had been treated with amoxicillin/clavulanic acid (Αugmentin f.c tabs 500+125 mg, GlaxoSmithKleine SA) (20 mg kg-1, BID, per os for 1 week) for chronic, intermittent, purulent nasal discharge. According to the history, the film-coated tablets were split to 1/10 portions using a pill splitter and the latter were administered without food or water. Physical examination revealed poor body condition, with a body condition score of 2/9 (ideal body weight corresponding to 5/9). The results of both complete blood count and serum biochemistry were within normal range, except for a mild increase in blood urea nitrogen levels. Serology testing for feline leukaemia virus and feline immunodeficiency virus was negative (Snap FIV/FelV, Idexx), and T4 serum levels were within normal range. Standard lateral thoracic radiographs showed severe dilation of the cervical and thoracic oesophagus (Figure 8), distended stomach due to gas, and marked presence of gas in the small and large intestine. Oesophagoscopy was performed using a flexible video endoscope (Pentax EG-1690K, 5.4 mm distal end external diameter, 2.00 mm forceps channel, 1,010 mm working length). No lesions consistent with oesophagitis were detected, and a stricture of <1.5 mm was identified (Figure 9). Repeated efforts to resolve the stricture with dilation balloons were unsuccessful, and in the final attempt the oesophageal wall ruptured. The owner refused surgical reconstruction of the oesophagus and placement of a gastrostomy tube, and the cat was euthanised.
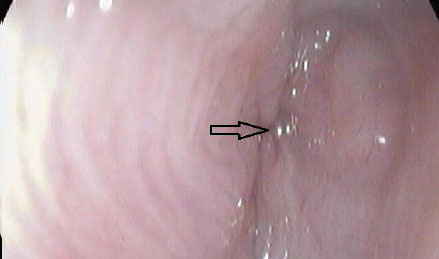 |
Figure 9: Interesting case 3. Endoscopic appearance of the oesophageal stricture. The arrow marks the stricture site, which gives an impression of atresia due to the severity of the stricture. |
Discussion
Drug-induced oesophagitis (DO) is a well-documented pathological condition in people, with over seventy drugs implicated in the aetiopathogenesis, although no strictures have been reported.7,19 Antimicrobials have been implicated in 50% of published cases, in which any other anatomical or functional abnormality of the oesophagus had been excluded. Dry swallowing of drugs, especially just before rest, can increase the risk for gastroesophageal reflux, especially considering that sleep reduces the production of saliva and swallowing frequency.8,19
The incidence of DO and BOS in pets is not known. Several published reports have implicated oxytetracycline, doxycycline and clindamycin in cats in contrast to dogs, for which no confirmation is evident in the literature.5,6,8,9,10 Prolonged contact of the oesophageal mucosa to a drug and its direct corrosive effect are the main causes of DO and BOS, whereas parenteral administration of the same drugs does not have the same result. The intrinsic tissue resistance of oesophageal mucosa to the offending drugs reduces the risk of oesophagitis. Oesophageal mucus bicarbonates, multiple layers of oesophageal epithelium, strong cell/cell connections, intracellular lipids, glycoproteins, mucosal perfusion, prostaglandins and leukotriene metabolism products contribute to tissue resistance.3
Doxycycline hydrochloride licensed for companion animals in the form of tablets (Ronaxan, Merial) forms an acidic solution in the neutral, under normal circumstances, oesophageal pH, and its corrosive, ulcer-forming effect in the feline oesophageal mucosa has been experimentally documented.6 Furthermore, doxycycline accumulation in the basal membrane of the oesophageal squamous epithelium has been noted, revealing another potential side effect of this drug.19 Clindamycin does not alter oesophageal pH, thus the aetiopathogenetic mechanism of DO and BOS is different.7 Drugs like clindamycin, that cause minimal mucosal irritation may become problematic if transit time through the oesophageal lumen and duration of contact with the oesophageal mucosa increase. The pharmaceutical formulation (capsule), dry swallowing, oesophageal hypomotility due to systemic disorders or dehydration at the time of administration are intrinsic factors in the aetiopathogenesis of side effects attributed to this drug.8 In people, there have been reports of DO associated with amoxicillin/clavulanic acid with an aetopathogenetic mechanism similar to that of clindamycin. There are no such reports in cats.
Studies have indicated that dry swallowing of capsules or tablets by cats can result in the drugs being retained in the cervical oesophagus (88%) or in the oropharynx (8%) for a duration of 30-240 seconds. 20 In a different study, more than 50% of capsules administered to healthy cats were trapped in the cranial thoracic oesophagus.9 A comparative study demonstrated that flavour-coated capsule/tablet administration with a pill gun (FlavoRx Pill Glide, FLAVORx, Columbia, Md.) or the offering of treats with drug pockets (Greenies Pill Pockets, Nutro Products, Franklin, Tenn.) can ensure an average oesophageal transit time of less than 60 seconds.4 Findings in all three cats of this case series (drug ingestion by dry swallowing, manifestation of clinical signs 3-16 days later, stricture site) are consistent with what is found in the literature.
In IC2, it should not be overlooked that short-lasting general anaesthesia was used as part of the surgical management of the abscess. In one study, the use of laryngeal mask airway device in 40 cats, 12-15 weeks of age, in which induction and maintenance of anaesthesia was obtained with isoflurane, resulted in GDOR in 50% of cats, compared to 22% of cases with endotracheal intubation.21 Although the anaesthetic process was brief, maintenance was accomplished via anaesthetic mask and there was no manipulation of intra-abdominal structures, it cannot be excluded that anaesthesia could have contributed to oesophagitis and stricture formation. Oesophagitis due to GDOR and subsequent stricture formation commonly occur in the segment of the thoracic oesophagus that is located 5-10 cm cranial to the lower oesophageal sphincter,3 in contrast to IC2, in which the site of the stricture in the cranial thoracic oesophagus at level of the heart base is consistent with DO and BOS due to clindamycin administration.
In IC3, according to the history, the onset of regurgitations followed the administration of amoxicillin/ clavulanic acid in the form of film-coated tablets for human use. Tablet coating is intended to preserve a drug until it reaches the proper absorption site in the intestinal tract, to protect the mucosal tissues from any irritant effect, and to regulate drug release at the same time.19,22 Cutting the tablets, giving formulations intended for human use, combined with dry swallowing are believed to have resulted in DO and BOS in IC3. The chronic recurrent nasal discharge in IC3 could have been a clinical manifestation of feline calicivirus infection, but this was not confirmed by laboratory testing. There are reports of ulcerative oesophagitis due to calicivirus in cats,3 but in such cases ulcerative glossitis/stomatitis is simultaneously present, along with diffuse oesophageal lesions and more severe clinical signs, none of which were observed in IC3.
In people, a correlation has been reported between old age and risk of medicine retention in the oesophagus.19 No such correlation has been found in cats, in this study or in the literature.10,12,18
Standard lateral radiographs rarely reveal the stricture site, especially in cases of multiple strictures,13 thus necessitating barium series and endoscopy in order to confirm the diagnosis.16 In IC1, the stricture site was evident after the administration of a contrast agent, in IC2 the lateral thoracic radiograph was normal, whereas in IC3 the lateral thoracic radiograph directly contributed to the diagnosis, revealing the oesophageal dilation cranial to the stricture site. Therefore, a combination of imaging modalities is necessary in order to obtain a final diagnosis. Treatment of oesophageal strictures is achieved by surgery, bougienage wax, dilation balloons, the distal end of the endoscope and stent placement.2,3,5,9,10 Restoration of oesophageal lumen diameter by dilation balloons is the method of choice as radially directed rather than shearing axial forces are exerted on the oesophageal lumen, minimising the risk of oesophageal rupture. Potential complications during the dilation procedure include oesophageal rupture (mainly in cats), mild tissue injury and oesophageal wall bleeding.15,16 In the present study dilation balloons was the method of choice. Nevertheless, oesophageal rupture still occurred in IC3.
The total number of dilation sessions can vary and it depends on the initial size of the stricture, the “aggressiveness” of the dilation procedure manipulations, the tissue responses of the affected animal and the chronicity of the stricture.16 In IC1 and IC2, 12 and 3 sessions were required respectively, when the average sessions required in cats range from 4-6.10 A higher number of sessions than what is reported in the literature as the maximum (8-11 sessions)4,8,9,16 should be the method of choice in the absence of alternative techniques such as stent placement.
In conclusion, the risk of DO and BES in cats should be taken into consideration by the clinician and it should not be underestimated. Any drugs in the form of capsules or tablets should be followed by food or 5-6 ml of water swallowing.20 These guidelines should apply to all drug categories that are orally administered in solid form to cats, considering the wide range of drugs that have been implicated for DO in humans.
References
1. Burk RL, Zawie DA, Garvey MS. Ballon catheter dilation of intramural esophageal strictures in the dog and cat: a description of the procedure and a report of six cases. Semin Vet Med Surg (Small Anim) 1987:241- 247.
2. Tams TR. Diseases of the esophagus. In: Handbook of Small Animal Gastroenterology. 2nd ed. Elsevier Science Saunders: Missouri, 2003, pp.118-158.
3. Ράλλης ΤΑ. Νοσήματα του οισοφάγου. Γαστρεντερολογία του σκύλου και της γάτας, 2η εκδ. University Studio Press: Θεσσαλονίκη, 2006, σελ.79-106.
4. Little SE. Diseases of the Esophagus. In: The Cat .Clinical Medicine and Management, Elsevier Saunders, St.Louis, Missouri, 2012, pp.443-446.
5. Adamama-Moraitou KK, Rallis TS, Prassinos NN, Galatos AD. Benign esophageal stricture in the dog and cat: a retrospective study of 20 cases. Can J Vet Res 2002, 66:55-59.
6. Carlborg B, Densert O. Esophageal lesions caused by orally administered drugs - an experimental study in the cat. Eur Surg Res 1980, 12:270-282.
7. Jaspersen D. Drug-induced oesophageal disorders: pathogenesis, incidence, prevention and management. Drug Saf 2000, 22:237-249.
8. Beatty JA, Swift N, Foster DJ, Barrs VR. Suspected clindamycinassociated oesophageal injury in cats: five cases. J Feline Med Surg 2006, 8:412-419.
9. German AJ, Cannon MJ, Dye C, Booth MJ, Pearson GR, Reay CA, Gruffydd-Jones TJ. Oesophageal strictures in cats associated with doxycycline therapy. J Feline Med Surg 2005, 7:33-41.
10. McGrotty Y, Knottenbelt C. Oesophageal stricture in a cat due to oral administration of tetracycline. J Small Anim Pract 2002, 43:221-223.
11. Glanemann B, Hildebrandt N, Schneider MA, Moritz A, Neiger R. Recurrent single oesophageal stricture treated with self-expanding stent in a cat. J Feline Med Surg 2008, 10:505-509.
12. Melendez L, Twedt D, Wright M. Suspected doxycyclin-induced esophagitis with esophageal stricture formation in three cats. Feline Practice 2000, 28:10-12.
13. Zawie DA. Esophageal strictures. In: Current Veterinary Therapy X. Small Animal Practice. Kirk RW, Bonagura JD eds. WB Saunders: Philadelphia, 1989, pp.904-906.
14. Weyrauch EA, Willard MD. Esophagitis and benign esophageal strictures. Compend Contin Educ Pract Vet 1998; 20:203-211.
15. Willard MD, Weyrauch EA. Esophagitis. In Current Veterinary Therapy XIII. Small Animal Practice. Kirk RW, Bonagura JD eds, WB Saunders: Philadelphia, 2000, pp.607-610.
16. Harai BH, Johnson SE, Sherding RG. Endoscopically guided balloon dilatation of benign esophageal strictures in 6 cats and 7 dogs. J Vet Intern Med 1995, 9:332-335.
17. Bisset SA, Davis J, Subler K, Degernes LA. Risk factors and outcome of bougienage for treatment of benign esophageal strictures in dogs and cats: 28 cases (1995-2004). J Am Vet Med Assoc 2009, 235:844-850.
18. Leib MS, Dinnel H, Ward DL, Reimer ME, Towell TL, Monroe WE. Endoscopic balloon dilatation of benign esophageal strictures in dogs and cats. J Vet Intern Med 2001, 15:547-552.
19. Kikendall JW, Friedman AC, Oyewole MA, Fleischer D, Johnson LF. Pill-Induced Esophageal Injury-Case reports and Review of the medical literature. Digestive Diseases and Sciences, 1983, 28:174-182.
20. Westfall DS, Twedt D, Steyn PF, Oberhauser EB, VanCleave JW. Evaluation of esophageal transit of tablets and capsules in 30 cats. J Vet Intern Med 2001, 15:467-470.
21. Sideri AI, Galatos AD, Kazakos GM, Gouletsou PG. Gastro-oesophageal reflux during anaesthesia in the kitten: comparison between use of a laryngeal mask airway or an endotracheal tube. Vet Anaesth Analg 2009, 36:547-554.
22. Channer KS, Virjee JP. The effect of size and shape of tablets on their esophageal transit. J Clin Pharmacol 1986, 26:141-146.
Corresponding author:
Vassiliki Stathopoulou
Plakentia Veterinary Clinic
31 Al. Panagouli Str. & 1-3 Viotias Str.
153 43 Agia Paraskevi
Tel.: +30 2106082308-9
Fax: 2106082343
e-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.



