Retrospective study of 56 cases
> Abstract
White Line Disease (W.L.D.) refers to hoof wall separation at the junction between the stratum medium and stratum internum of the epidermis that subsequently forms a cavity. This study included 56 horses with W.L.D. that were admitted to the Equine Unit, Companion Animal Clinic, Faculty of Veterinary Medicine, Aristotle University of Thessaloniki over the last 3 years. The cause of W.L.D. has been attributed to incorrect hot shoeing (overheated-dried out hoof) in 18 horses, overhydration of the hoof in 10 horses, dehydration of the hoof due to environmental factors in 6 horses, improper shoeing (“nail bind”- small or inappropriate horseshoe, contamination of nail holes) in 8 horses and combination of the above in 6 horses. Stall hygiene (stall bedding) and training ground were inappropriate in 48 cases. Disease affected the forelimbs, unilaterally or bilaterally in 39 (69.64%) horses and hindlimbs, unilaterally or bilaterally in 10 (17.85%) horses. In the remaining 7 (12.5%) horses forelimbs and hindlimbs were randomly affected. Therapeutically, debridement of the cavity, daily rinsing with aqueous solution eosin 2%, heart-bar shoe, biotin and rest were recommended. The majority of horses (91%) responded positively in the treatment protocol described above.
This retrospective study reveals the relatively high prevalence of W.L.D. in the region of Thessaloniki considering the fact that 15-20 horses were affected per year (4% of the total population), while a 20% present with secondary disease with guarded or poor prognosis.
> Introduction
White line disease (W.L.D.) affects quite often the horse’s hooves with or without the appearance of clinical symptoms.1 The etiology is not fully specified while disease appears to have widespread occurrence, and potentially can affect all breeds, ages and sexes.2
W.L.D. is defined as the separation of the hoof wall between the stratum medium and stratum internum, resulting in the creation of a cavity.2-9 The width of separation determines both treatment and prognosis.
Causative agents include fungi and bacteria2,9 entering the white line and eroding the hoof wall. This requires appropriate incubation conditions, facilitated by the presence of predisposing factors, mainly associated with moisture of the hoof and incorrect shoeing.2,9,11 However, in some cases, etiology is unclear, suggesting the presence of additional causative or predisposing factors.
Diagnosis is based on typical clinical signs and the presence of tympanic sound produced by the percussion of the hoof and confirmed by radiographic examination.2,7,9 Lameness rarely occurs and is usually absent in the uncomplicated forms of the disease. Therapeutically, exposure of the affected cavity and debridement are essential2,4,9 as much as the treatment of concurrent secondary injuries. Moreover, therapeutic shoeing in conjunction with supplements that promote hoof growth are suggested.2,4,6,9
The purpose of this study is to present cases with W.L.D. that were admitted to the Companion Animal Clinic, Faculty of Veterinary Medicine, Aristotle University of Thessaloniki, over the last 3 years (2009-2011). Epidemiology, etiology, clinical signs, diagnosis, treatment protocol and response are presented below.
> Clinical cases
Materials and Methods
This study included 56 horses with W.L.D. that were admitted to the Equine Unit of the Companion Animal Clinic, Faculty of Veterinary Medicine, Aristotle University of Thessaloniki during the last 3 years. More specifically, 5 stallions (8.47%), 25 geldings (44.64%) and 26 mares (46.42%) were examined. The majority of horses were Warmbloods 47 (83.92%), 3 (5.35%) of them Greek breed, 1 (1.78%) mixed breed while 5 were English Thoroughbreds. Their age ranged from 4 to 17 years.
Predisposing factors were determined in 48 horses while it remained unclear in the remaining 8 horses (14%). In those cases that predisposing factors were found, the disease was attributed to accidents related to hot shoeing (overheated, dried-out hoof) in 18 horses (32%), over-hydration of the hoof in 10 (17%), dehydration due to environmental factors in 6 (10%) horses, improper shoeing (“close nail”, small or inappropriate horseshoe, contamination of nail holes) in 8 (14%) and combination of the above in 6 (10%) horses. Stall living conditions (stall bedding) and training ground were inappropriate in all cases.
Disease affected one or both forelimbs in 39 horses (69.64%), one or both hindlimbs in 10 horses (17.85%), while 7 horses (12.5%) were affected in various combinations. During the initial clinical examination the farrier had already opened up the hoof wall in 39 horses (69.64%). Tympanic sound on percussion of the perioplium and presence of black spots at the level of white line after shoe removal were noted in the remaining 17 horses (30.36%). In 9 horses (16%) curvature of the toe was detected. During lameness examination, only 12 horses were lame (2/10 to 6/10).
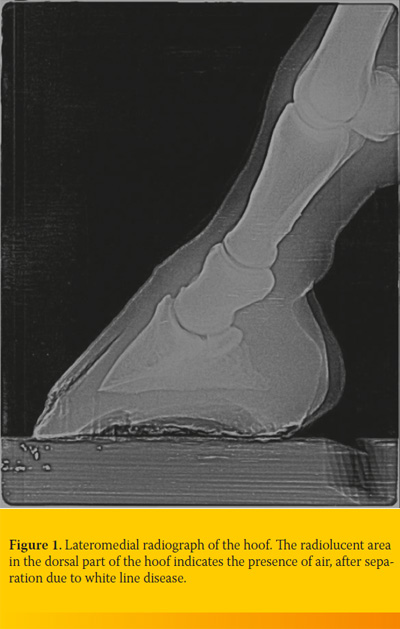 In the horses that the hoof wall was already open, diagnosis was based on the basis of clinical presentation and the width of cavity was further explored by passing a probe through the abscess combined with percussion on the peripheral limits using a hoof tester. In horses with intact hoof wall diagnosis was based on the presence of black discoloration of the white line after the removal of the shoe, tympanic sound production during the percussion of the hoof wall and radiographic examination (presence of air, wall separation) (Figure 1). Radiographs of the hoof were taken in 12 horses presented with lameness. Laminitis was diagnosed in 8 of these horses, while pedal osteitis of the forelimbs was identified in the remaining 4 horses.
In the horses that the hoof wall was already open, diagnosis was based on the basis of clinical presentation and the width of cavity was further explored by passing a probe through the abscess combined with percussion on the peripheral limits using a hoof tester. In horses with intact hoof wall diagnosis was based on the presence of black discoloration of the white line after the removal of the shoe, tympanic sound production during the percussion of the hoof wall and radiographic examination (presence of air, wall separation) (Figure 1). Radiographs of the hoof were taken in 12 horses presented with lameness. Laminitis was diagnosed in 8 of these horses, while pedal osteitis of the forelimbs was identified in the remaining 4 horses.
Treatment protocol was generally the same in the 44 sound horses. Initially, wide opening the cavity was performed followed by daily rinses with aqueous solution eosin 2% (Figure 2). Debride ment that included all the affected wall part was half-round shaped or rectangular. Debridement margins continued up to healthy hoof wall. In all cases hot shoeing was suggested. Fourteen horses were shoed with an egg-bar shoe9 and 30 horses with heart-bar shoe with or without silicone frog pads (Figure 3). To stimulate hoof growth, cornucrescine ointment was applied on the coronary band once daily for one week and the daily ratio was supplemented with biotin (20mg SID per os for at least one month).

Box rest for at least one month was recommended in 12 lame horses. Debridement was followed by application of heart-bar shoe. Isoxuprine hydrochloride (1mg/kg every 12 hours per os) was administered in 5 of 12 horses with lameness while biotin, eosin / iodine were administered in all horses. In addition, 8 horses with laminitis and 4 horses with pedal osteitis received additional treatment for each concurrent disease. Restoration of the normal hoof shape and, thus, complete rehabilitation ranged from 2 to 18 months (5 months on average).

Results
The treatment protocol described above had positive results in the majority of cases. Specifically, all 44 horses with no lameness resumed full work directly, despite the defects of the stratum corneum. Structural integrity of hoof wall restored the next 2 to 5 months, depending on the size of absence (3.5 months on average). Five of the 8 horses suffering from laminitis had clinical signs and radiographic findings suggestive to chronic laminitis with guarded prognosis. The remaining 3 horses with laminitis as well as the 4 horses with pedal osteitis recovered fully.
> Discussion
From outside to inside, hoof wall is comprised of the stratum corneum, the white line and the dermis. 1,10 Stratum corneum is the protective sheath of the sensitive structures of the hoof separated by the white line.1,10 White line is visible in the sole, which encloses peripherally, and microscopically consists of 3 layers; the outer, the middle and the inner layer.1,2,10 Dermis, including the primary and secondary laminae, is the functional suspensory system of the third phalanx.10
Causative agents of white line disease include a number of anaerobic bacteria and fungi, acting individually or in various combinations.2,7,8,9,11,12 The most frequently isolated pathogens are Gram- bacteria (Bacteroides, Fusobacterium) and Pseudoallsheria, Scopulariopsis and Aspergillus fungi.2 In this study identification of the responsible microorganisms was not possible, as in most cases wide opening of the hoof wall had preceded and, consequently, culture would not be representative, or the owners did not wish further investigation. In any case it has been proved that cultures in cases with W.L.D. formed mixed populations and did not facilitate the choice of treatment protocol.2 Responsible pathogens enter the hoof through small pinpoints and in combination with appropriate conditions, they use nutrients of the hoof wall to grow, proliferate and finally erode it. Requirements for incubation are increased temperature and humidity, lack of oxygen and light.3,11 This process relies on several predisposing factors, which when they act in combination, favor disease development.
Moisture of the hoof is the main predisposing factor, as described in literature and ascertained by this study.2,7,9,11,12 Conditions of high humidity make the stratum corneum an ideal substrate for microorganism growth, whereas, low humidity make the hoof brittle, potentially prone to cracksportals to microorganism entry. Maintaining the optimum moisture conditions depends on the care given by the owners, the stall bedding,2,7 the exercise conditions of the horse, the genetic potential and the shoeing technique. The fact is that the majority of horse owners use hoof care products (fat, oil, formalin) excessively, while stall conditions are unsuitable. Hot shoeing is a commonly used technique aiming to give a better shape, easier application of the horseshoe and to fight microorganisms of the sole. However, in this study, improper shoeing technique was frequent (18 horses), as prolonged contact with the heated up, glowing horseshoe led to overheating and drying out the hoof. Thus, humidity levels were off limits in 25 cases; 12 of which had soft hooves due to excessive moisture, 3 had canker and 10 had brittle and dried out hooves.
Additionally, shoeing alone can be an important triggering factor of the disease.2,12 Improper shoeing makes nail holes portals for microorganisms to enter and creates suitable conditions for colonization. In fact, two farriers noticed gray/ black coloured8,9 perimeter of nail holes, signs of disintegration and sepsis, in 8 horses that developed the disease at a later stage. Proper trimming at these points would probably have prevented W.L.D. In 8 horses the disease was considered to be the result of direct placement of the nail in sensitive structures (“nail prick”) or the use of a smaller size shoe, leading to mechanical separation of white line and secondary W.L.D. Poor conformation of the hoof is considered to be an important predisposing-etiologic factor,2,12 usually accompanied by reduced blood supply. Deviation from normal conformation is a result of either genetic predisposition or management factors. Genetically, it occurs with reduced frequency in foals with angular limb deformities (varus-valgus).4 This “condemns” the hoof in unnatural growth in all 3 axes. Management factors include frequency and technique of trimming. Ideally the hoof, with minor exceptions, should be trimmed every 45 days. Trimming intervals exceeding 50-55 days lead to overgrowth of the toe and abnormal distribution of forces in the standing or the walking horse. In these cases, therefore, appropriate conditions are created for the development of W.L.D., mechanically5 and pathophysiologically (decreased blood supply). Although the argument described above seems pathogenetically reasonable, this study produced no data to support it. In any case, balance of the hoof is of paramount importance.
In addition to moisture and morphology of the hoof, preexisting pathological conditions have been implicated as predisposing factors. Although literature data implicate sepsis, cracks, hoof abscess, laminitis and foreign bodies, 2,4,11,12 this study revealed no clear correlation. Predisposing factors, such as necrotic pododermatitis (canker) and entrapped foreign body in the sole are doubtful.5,6
Although the role of these etiologic-predisposing factors is indisputable, full understanding of W.L.D. is questionable while in some cases it appears without any of the factors described above being present or noticed.
Pathogenetically, W.L.D. has been described as a keratolytic process of the solar surface, caused by microorganisms that progressively separate the hoof layers.2 At an early stage, the cavity formed by this process is not accompanied by overt clinical signs; however it may lead to serious and irreversible orthopedic injuries.
This retrospective study of W.L.D. over the last 3 years (2009-2012) reveals the relatively high prevalence of the disease in the region of Thessaloniki as 4% of the total population is affected per year (15-20 horses per year) and a 20 % develop secondary disease with guarded or poor prognosis.
Epidemiological data indicate that there is no correlation between the disease and sex or age of the horses. Although it seems that the majority of horses belonged to improved breeds (Warmblood, English Thoroughbred), this conclusion is questionable probably due to the population of horses in the region of Thessaloniki that are admitted to Companion Animal Clinic.
W.L.D. occurred significantly more often in forelimbs without documented aetiopathogenesis. This was probably associated with the high prevalence of orthopedic injuries in forelimbs as they bear heavy load (65% of body weight).3
Clinically, the majority of horses showed no signs of pain or lameness. The cavity of hoof wall extended from a few centimeters to the largest part of total hoof wall, while depth did not exceed the inner layer of the white line.
Diagnosis was easy and was based on clinical examination (separation of hoof wall-visible the inner layer), percussion (air interference-tympanic sound), exploration of the cavity with probe and radiographic examination (detachment of hoof wall-presence of air).2,3,9 Although the widely used diagnostic methods described above, in cases with W.L.D., they are implemented days or even months after the initial infection, which confirms the latent nature of disease and emphasizes the need for greater vigilance by the veterinarian and the farrier. These elements, visible only after trimming is deviation of white line and the presence of grey/black erosions along it.2,8 As aforementioned, farriers had noticed these lesions in a number of horses. These are the first colonies of microorganisms and direct treatment eliminates the possibility of further development.
Although treatment and rehabilitation in cases with W.L.D. are prolonged, prognosis is generally favorable. Hoof wall resection is the cornerstone of treatment.2,4,7,9 The hoof is trimmed, cleaned and the undermined hoof wall is removed. Ideal resection includes all the affected hoof wall, extending to the limit of healthy margins; however, mechanical stability of the hoof should be borne in mind. This is achieved by not excising more than 2/3 of the total hoof height. In cases where the cavity is extensive, opening up holes in the proximal part of the hoof provides a good alternative. The shape of the cavity and holes are determined by both the veterinarian and the farrier so as to obtain sufficient aerobic conditions, maintaining the stability of the hoof at the same time.
Heart-bar shoe is the shoe of choice.3,4,8,9 Lowering the heels and reducing the length of toe transfers the weight of horse backwards and, hence, reduces the load of the undermined area and the third phalanx.8,9 This method was chosen in 30 horses suspected for instability of the hoof and secondary laminitis, due to extensive lesions and excess removal of the hoof wall. Silicone pads and support clips were used in the majority of horses.2 Specifically, silicone pads offer shock absorption while clips stabilize the shoe since, in some cases, hoof wall is not sufficient for placing of the nails. However, an egg-bar shoe was considered a safe choice in mild cases,7 when there is no risk of complications. Generally, the type, shape and size of the shoe is determined on a case-by-case basis, under the guidance of veterinarian and farrier together.
It is compulsory to clean the undermined area daily and treat it with antiseptic solutions.2,4,9 The literature indicates that wire brush is the most effective way to thoroughly clean the hoof.2 Although hot shoeing was a main causative factor in this study, it is generally considered as an excellent technique for fighting microorganisms, provided that it is properly applied. It was recommended in horses with early-stage lesions.
Pharmaceutically, substances that accelerate hoof growth reduce recovery time. These are biotin supplements (biotin, lysine, metheionine, zinc, cystine, cysteine), vesicants of the coronary band7 (cause vasodilation locally) and isoxsuprine hydrochloride (peripheral vasodilator). Biotin administration is considered to be the most effective treatment to increase hoof growth.8,9 However, this study suggests that along with per os administration of biotin, coronary band vesicants and isoxsuprine hydrochloride led to rapid hoof growth compared to those treated with biotin alone.
Regarding training regime, the patient was able to work normally provided that mechanical stability of hoof was sufficient. However, there were exceptions; such as horses with excessive resection and, hence, increased likelihood for hoof imbalance. 7 As a result, box rest or mild/controlled exercise was suggested.
It is worth mentioning that 3 horses with mild lesions subsequently presented acute lameness (2/10) that resulted from the sensitization of toe dermis from mechanical injuries after removing of the periople. These injuries, caused by either kicking against the stable door (2 of 3 horses) or jumping (third horse) were treated with non-steroidal anti-inflammatory drugs (phenylbutazone 4 mg/kg per os SID for 5 days) and rest. Lameness resolved 5 days after treatment, a fact that did not change either prognosis or treatment.
Horses with secondary lesions received more aggressive therapy. The most common complication of W.L.D. is laminitis2,3 (8 horses) due to the destruction of laminae caused by the change of forces applied to them. Other complications are pedal osteitis2,3,9 (4 horses) caused by increased loading or rarely due to extent of inflammation in the dermis, and, finally, deep uncontrolled septic infection of dermis. In this study, late diagnosis and lack of monitoring of horses were identified as the causes of complications. It is worth mentioning that excessive debridement led to laminitis in two horses. These cases caused a re-evaluation of cavity margins and therapeutic approach. It is commonly reported that the automatic extension of the cavity and, therefore, infection of the coronary band is associated with particularly poor prognosis.8,3 Fortunately, this was not supported by the data of this study.
In complicated cases, horses were presented with lameness, pain and radiographic abnormalities proportional to the damage suffered. Diagnosis of W.L.D was made as described above and, therapeutically, corrective shoeing was necessary, as well as aforementioned supplements for a long period of time. Shoeing with polymerized acrylic material rather than nails2,9 is proposed by other authors. This method is safer as creating nail holes is avoided, and also technically easier as in several cases hoof wall is inadequate for placing the nails. The main disadvantage of these shoes is that they do not stay-on as securely as nail shoes. This method was not implemented in cases described above. Treatment for secondary lesions was also carried out. Horses received box rest until deemed necessary. Particular attention has to be paid to eliminate predisposing factors, preventively and therapeutically, to minimize the chances of reinfection and relapse.
Placement of acrylic material in the cavity after obtaining sterile conditions is an alternative and immediate treatment.3,9 Acrylic material replaces hoof defect, restoring natural protection of deep hoof structures and mechanical stability. Despite the fact that this method is reported, it was not applied in the current study as it is technically difficult and poses serious risks. The main risk is mechanical entrapment of microbes with concurrent creation of anaerobic environment. Moreover, acrylic material attenuates the surrounding hoof wall, delaying hoof repair.
Monthly measurements are taken to determine the response to treatment and hoof growth rate. A practical way is marking the hoof just below the coronary band with a permanent marker and monitoring the growth.
The diagnostic and therapeutic approach described above led to satisfactory clinical response. It is readily understood that the chronic nature of the cases of this study was not related to pathogenetic mechanisms but to delayed detection and treatment due to the latent nature at early stages. According to the progress of cases described above it is concluded that a clinician treating a horse with W.L.D. should focus on early detection of clinical signs before the occurrence of complications. Substantial cooperation among the owner, the veterinarian and the farrier is the most important factor for a successful outcome.
> References
1. Michail SG . Skin: Histology. Kyriakidis Brothers. 2nd Edition. 2004, 249-251.
2. O’Grady SE. A fresh look at white line disease. Equine Vet Educ 2011, 23(10): 517-522.
3. Stashak TS, Hill C, Klimesh R, Ovnicek J. White line disease. In: Adam’s Lameness in Horses. 5th edn. Stashak TS (ed). Lippincott, Williams and Wilkins: Philadelphia, 2002, pp. 1117- 1118.
4. Wildestein MJ. Balance of the hoof. In: Equine Medicine and Surgery. Colahan PT, Mayhew IG, Merritt AM, Moore JN (eds). 5th edn. Mosby: Missouri, 1999, pp. 1424.
5. Moyer WA, Colahan PT. Canker. In: Equine Medicine and Surgery. Colahan PT, Mayhew IG, Merritt AM, Moore JN (eds). 5th edn. Mosby: Missouri, 1999, pp. 1544-1546.
6. Ross MW, Dyson SJ. Trauma to the Sole and Wall. In: Diagnosis and Management of Lameness in the horse. 2nd edn. Elsevier Saunders: United States, 2011, pp. 312-313.
7. Stashak TD. White line disease. In: Practice Guide to Lameness in the Horses. Lippincot Williams: Philadelphia, 1995, pp. 219-220.
8. Pollitt CC. Disorders and Diseases of the Hoof Wall In: Color Atlas of the horse’s foot. Mosby: Barcelona, 1995, pp. 109-118.
9. McDonald MH, Kannegieter N, Peroni JF, Mergy WmE. White line disease. In: The equine Manual. Higgins AJ, Snyder JR (ed). 2nd edn. Elsevier Saunders: Missouri, 2006, pp. 984-985.
10. 10. Denoix JM. The equine foot In: The equine distal limb. Manson Publishing: Bercelona, 2000, pp. 1-5.
11. Shettko DL. The Equine Geriatric Foot In: Equine Geriatric Medicine and Surgery. Bertone J (ed). Elsevier Saunders: Missouri, 2006, pp. 217-221.
12. Rooney JR. White line disease. In: Equine Podiatry. Floyd AE, Mansmann RA (ed). Elsevier Saunders: Missouri, 2007, pp. 70-71.



