Vasileia Angelou DVM, MSc, Dimitra Karatzia, George Kazakos DVM, PhD
Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
MeSH keywords:
conscious sedation, dog, gastric dilatation, neoplasms
Abstract
Anaphylaxis is a severe life-threatening hypersensitivity reaction, which can be triggered by exposure to a variety of antigens. In anaphylaxis, inflammatory mediators are released resulting in the onset of clinical signs. In small animal veterinary practice, the main clinical manifestations are observed in the skin, gastrointestinal tract, cardiovascular system, respiratory system, nervous system and eyes. This variety of clinical signs depends on the route of antigen exposure and on how abrupt the onset of clinical signs was. The diagnosis of anaphylaxis is not based on laboratory methods but on cautious observation of clinical signs and detailed history, which will assist in locating the initiating cause. However, identification of the initiating cause during the perioperative period can be particularly challenging (i.e. when a combination of drugs has been administered). Emergency treatment is frequently based on timely administration of adrenaline in the appropriate dose and by the appropriate route. Furthermore, supportive treatment may include the administration of antihistamines, corticosteroids and bronchodilators whereas oxygen and intravenous fluids are a mainstay of treatment especially when anaphylactic shock has already occurred. Prognosis depends on this variety of clinical signs and the time elapsed from the onset of symptoms to the initiation of treatment. Prevention is not only challenging but unattainable in a clinical setting, based only on allergen avoidance. Even though systemic anaphylaxis can prove fatal even within minutes, studies regarding this subject are scarce. Still nowadays, management of systemic anaphylaxis remains a challenge for the veterinary clinician.
Introduction
Anaphylaxis is a severe, potentially life-threatening hypersensitivity reaction which occurs abruptly after exposure to an antigen (Hepner & Castells 2003, Sampson et al. 2006, Carter et al. 2011). It was first described in 1902 from Portier and Richet, who concluded that the body is initially sensitized to an allergen and the clinical signs of anaphylaxis emerge when it is exposed a second time to the same allergen (Portier & Richet 1902). Main manifestations, in both human and veterinary medicine, include clinical signs originating from different body systems, mainly dermatological, gastrointestinal, cardiovascular, respiratory and less often neurological and ophthalmic (Hepner & Castells 2003, Dowling 2014). Even though it seems to be a fairly common disorder in dogs and cats, searching the PubMed database with the keywords “anaphylaxis, dogs, cats” resulted in only 56 publications. Most of these studies regard anaphylaxis as a response to vaccinations, environmental factors, insect bites as well as anaesthetic drugs, antibiotics etc., whereas using the keywords “anaphylaxis, anesthesia, dogs” reduced the case report number that was recovered to 18 in total. Especially during the perioperative period in which multiple drugs are administered, it can be challenging to associate a response to a particular drug, even more so with enough certainty that this can be published in an approved journal (Armitage-Chan 2010, Dowling 2014). In general, both in veterinary and human medicine, despite the various severe clinical signs which can be caused by anaphylaxis in many different body systems, the definitive diagnosis of an anaphylactic reaction is challenging. Therefore, in the past few decades an effort has been made to define specific and clear criteria which will regard every possible episode (Sampson et al. 2006, Shmuel & Cortes 2013). Management of such cases is even more difficult when they result in complications during the perioperative period.
The aim of this study is to review the literature on anaphylaxis in dogs and cats focusing in pathogenesis, aetiology, clinical manifestations, diagnosis and treatment, and emphasizing on the perioperative period.
Pathogenesis
Hypersensitivity reactions have been classically classified in four categories: type I or immediate mediated by IgE immunoglobulins, type II or cytotoxic mediated by IgG and IgM immunoglobulins, type III caused by immune complex formation (antigen-antibody) and type ΙV or delayed-type hypersensitivity mediated by T-lymphocytes (Dowling 2014).
Even though anaphylaxis has been attributed to type I hypersensitivity reaction, a non-immunologic form of anaphylaxis has also been identified both in humans and companion animals (European Academy of Allergy and Clinical Immunology). This form of hypersensitivity has been called anaphylactoid reaction, and it is not mediated by IgE immunoglobulins and previous exposure to the initiating cause is not necessary in order to trigger this response (Armitage-Chan 2010, Shmuel & Cortes 2013, Dowling 2014). However, this is not an entirely accurate term, considering that IgE receptors may be activated by a different antigen than the one that resulted in clinical signs during the first anaphylactic episode (Armitage-Chan 2010). As previously clarified, the pathogenesis of the non-immunologic anaphylactoid reaction has not been elucidated yet. The observation that is commonly caused by intravenous infusions of drugs, especially with clinical manifestations matching the rate and speed of the infusion, has led to the revelation that it can be triggered even by changes in ion concentrations in the transmembrane potential or osmolality transitions and/or receptor stimulation which will indirectly cause the release of histamine (Descotes et al. 2007, Armitage-Chan 2010). On the other hand, these two forms cannot be clinically differentiated (Descotes et al. 2007, Armitage-Chan 2010). However, anaphylaxis, either immunological/allergic or non-immunological/ non-allergic, results in the activation of mast cells, the release of basophils in the systemic circulation and the release of chemical anaphylaxis mediators including mostly histamine, leukotrienes, platelet-activating factor (PAF) and prostanglandin D2 (PGD2) (Hepner & Castells 2003, Peavy & Metcalfe 2008, Khan & Kemp 2011, Shmuel & Cortes 2013, Dowling 2014). The release of such inflammatory mediators occurs in seconds to minutes and cytokine synthesis is initiated within hours (Dowling 2014). Finally, it is unknown whether cytotoxic response (type ΙΙ), immune complex responses (type ΙΙΙ) or delayed- type hypersensitivity mediated by T-lymphocytes (Type IV) can result in anaphylaxis (Dowling 2014).
Aetiology
Any agent in general may activate mast cells and basophils and cause anaphylaxis (Hepner & Castells 2003, Simons 2009). Main agents that may cause anaphylaxis include food, snake or insect bites, vaccinations, as well as environmental factors such as intense exercise, extreme cold or heat (Jackson et al. 2003, Shmuel & Cortes 2013, Dowling 2014). In animals as well as in humans, drugs are a wide category of agents responsible for anaphylaxis. Most of these agents are drugs administered during the perioperative period (e.g. non-steroidal anti-inflammatory drugs, antibiotics and corticosteroids) (Schaer et al. 2005, Dowling 2014). Anaesthetics are also a common cause of immunologic or non-immunologic anaphylaxis (Armitage-Chan 2010). Drugs such as morphine and pethidine have been known to result in release of histamine, especially during intravenous infusions; therefore, this route is not recommended (Armitage-Chan 2010). In dogs and cats anaphylaxis has been previously reported due to thiopentone (Mason 1976, Burren & Manson 1986), acepromazine (Meyer 1997), xylazine-ketamine (Raptopoulos et al. 1993), alfaxalone (Haworth et al. 2019), pethidine (Schachter 1952), medetomidine (Viscasillas et al. 2011), free morphine and ropivacaine after epidural administration (Threlfall et al. 2012), as well as rocoronium (Küls et al. 2016), paramagnetic contrast agents (Pollard & Pascoe 2008, Girard & Leece 2010) and other drugs administered during the perioperative period. Other causes include fresh blood or blood product transfusions, human albumin, colloids and chemotherapeutic agents such as L-asparaginase (Mertes & Laxenaire 2004, Mathews 2006, Francis et al. 2007, Blake et al. 2016). Anaphylactoid reaction caused by intravenous infusion of vitamin Κ1 is also worthy of note (Mi et al. 2014). There are unanswered questions over the use of muscle relaxants in animals that have been implicated in anaphylactoid reactions in humans during anaesthesia. However, in animals there are not enough reports for conclusions to be drawn, except a single case report in a dog that presented with anaphylactoid reaction during anaesthesia in which intravenous rocuronium was implicated (Küls et al. 2016). Even though neuromuscular blocking agents have been considered relatively safe to use, possibly due to a lack of reports to the contrary, special care should be provided especially in animals with a history of anaphylaxis (Jones 1992, Küls et al. 2016). Moreover, anaphylaxis during surgery has been reported in a case of lung lobectomy in a heartworm-infected dog possibly due to accidental dissection of the parasite (Carter et al. 2011). The biggest problem during the perioperative period with anaesthetics, antibiotics, analgesics and other drugs is that they are administered in various combinations. Therefore it is difficult to identify the causative agent and consequently case reports regarding this matter are scarce (Armitage-Chan 2010). It is also worth mentioning that there might be cases of anaphylaxis which cannot be associated with a known causative agent or event (Choo et al. 2010).
Clinical manifestations
Clinical signs depend on animal species, previous sensitization to an allergen, amount of the allergen and route of exposure to the allergen (Schaer et al. 2005, Shmuel & Cortes 2013). In the clinical setting, dogs are most commonly admitted with hypotension and haemorrhagic diarrhoea, whereas respiratory signs are uncommon (Quantz et al. 2009, Shmuel & Cortes 2013). In cats, even though case reports are scarcer, the main manifestation of anaphylaxis is respiratory distress (Litster & Atwell 2006, Moore et al. 2007, Hume-Smith et al. 2011, Dowling 2014). The non-intestinal clinical signs emerge abruptly and are usually more severe compared to localized external exposure, and they include mostly cardiovascular and respiratory manifestations (Schaer et al. 2005, Kemp et al. 2008). When the responsible allergen has been inhaled, the intestine is mostly involved, combined with skin reactions and bronchoconstriction and manifestations from the nasal mucosae (Dowling 2014). Localised external contact with the allergen may lead to dermatological and systemic signs (Dowling 2014). The speed with which the inciting allergen is administered is also of importance as well as whether or not the allergen has been diluted (Schaer et al. 2005, Guedes et al. 2006, Armitage-Chan 2010). The time elapsing between exposure to the allergen and onset of clinical signs is associated with the severity of anaphylaxis both in humans and small animals (Khan & Kemp 2011, Shmuel & Cortes 2013, Dowling 2014). The more abruptly clinical signs manifest, the more severe they will be (Kemp et al. 2008, Khan & Kemp 2011). Clinical signs usually occur within 5-30 minutes, however in certain cases several hours may pass until clinical signs are evident (Choo et al. 2010, Moore & HogenEsch 2010, Khan & Kemp 2011). In human medicine a biphasic reaction has been described during which after the initial clinical signs have been controlled, they may reappear within 1 to 72 hours (Sampson et al. 2006, Simons et al. 2011). Other than biphasic anaphylaxis, delayed type III hypersensitivity reaction has been observed in dogs with manifestation of clinical signs up to days or weeks after exposure to the allergen (Francis et al. 2007). In particular, there is a paper regarding the intravenous infusion of human albumin in 6 dogs which evidenced clinical signs after 5-13 days (Francis et al. 2007) and another one regarding a case of a dog that manifested clinical signs on the third day after antivenom (anti-ophidic serum) had been administered (Berdoulay et al. 2005).
Clinical signs (Table 1) usually include dermatological, gastrointestinal (predominantly in dogs), cardiovascular, respiratory (predominantly in cats), neurological and ophthalmic manifestations (Shmuel & Cortes 2013, Dowling 2014). Dermatological signs are not as frequent in dogs and cats as they are in humans. These signs can be part of the initial stage during the manifestation of anaphylaxis and may be included in a delayed response or they may not be noted at all (Sampson et al. 2006, Quantz et al. 2009). These cutaneous responses are frequently very mild or may not be readily observed due to the fur coat or skin pigmentation (Quantz et al. 2009). Dermatological signs include erythema, pruritus, urticarial and angioedema which is the most commonly observed skin manifestation of anaphylaxis and predominates in dogs (Armitage-Chan 2010, Shmuel & Cortes 2013, Dowling 2014). Pruritus is usually generalised whereas angioedema is most commonly observed on the head, limbs and genitalia (Francis et al. 2007, Girard & Leece 2010, Dowling 2014).
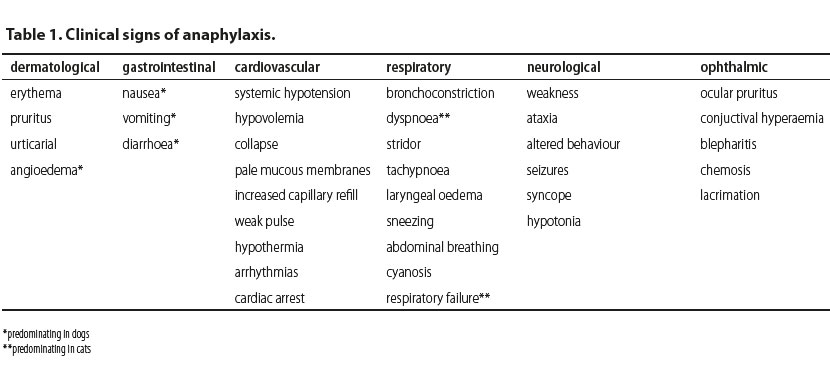
Clinical signs from the gastrointestinal tract are nonspecific, and include nausea, vomiting and diarrhoea (Shmuel & Cortes 2013, Dowling 2014). In dogs, gastrointestinal signs predominate and are usually caused by portal hypertension (Lautt & Legare 1987, Richardson & Withrington 1987, Quantz et al. 2009). There is a published paper which reports that increased alanine aminotransferase and abnormalities in gallbladder imaging during abdominal ultrasonography have been attributed to anaphylaxis (Quantz et al. 2009).
The main manifestation of cardiovascular compromise in both dogs and cats is systemic hypotension due to peripheral vasodilation and increased vascular permeability caused by inflammatory mediators during anaphylaxis. Increased vascular permeability also leads to fluid extravasation from the intravascular to the extravascular space resulting in hypovolemia. If prolonged, this can lead to circulatory collapse (shock) which is further characterized by pale mucous membranes, increased capillary refill time, weak pulse and hypothermia. Tachycardia is anticipated due to hypovolemia, however sometimes bradycardia is observed due to stimulation of the Βezold-Jarich reflex which is activated in cases of hypovolemia (Armitage-Chan 2010). As well, cardiac arrhythmias or even cardiac arrest may occur (Shmuel & Cortes 2013).
Main respiratory signs in small animals include bronchoconstriction, dyspnoea, coughing, stridor and tachypnoea (Armitage-Chan 2010, Shmuel & Cortes 2013, Dowling 2014). Moreover, there may be laryngeal oedema, sneezing and in severe cases abdominal breathing, cyanosis and respiratory failure (Shmuel & Cortes 2013, Dowling 2014). In cats, dyspnoea is the first sign of anaphylaxis which can be caused by laryngeal and pharyngeal oedema, bronchoconstriction and increased mucus secretion (Hume-Smith et al. 2011, Dowling 2014). Humans with a history of respiratory disease may develop more severe respiratory signs even though it has not been proven that these patients are more susceptible to anaphylaxis (Shmuel & Cortes 2013).
Neurological and ophthalmic signs are rarer in clinical practice, compared to the symptoms described above. Neurological signs in small animals include generalised neuromuscular weakness, ataxia, changes in behaviour, seizures, syncope or hypotonia which are related to hypotension (Quantz 2009, Hume-Smith et al. 2011, Shmuel & Cortes 2013, Dowling 2014, Rostaher et al. 2017). Ophthalmic signs, both in dogs and cats may include ocular pruritus, conjuctival hyperaemia, blepharitis, chemosis and lacrimation (Hume-Smith et al. 2011, Shmuel & Cortes 2013).
Diagnosis
Clinical diagnosis of anaphylaxis can be challenging, not only due to the lack of specific pathognomonic clinical signs, but also due to the wide spectrum of clinical manifestations (Shmuel & Cortes 2013). There are criteria in humans that need to be met in order to diagnose anaphylaxis (Sampson et al. 2006). However, further studies are required in order to use such criteria in veterinary medicine (Shmuel & Cortes 2013). In small animal practice, information from the clinical history can be very useful, such as previous reactions to vaccinations, food, insect bites as well as recent transfusions or administration of antibiotics, anaesthetic agents, and other drugs as well as the time between the exposure to a known antigen and symptoms manifested (Shmuel & Cortes 2013). Such information combined with possibly abrupt manifestation of clinical signs can often be the only evidence of anaphylaxis (Sampson et al. 2006, Shmuel & Cortes 2013). Allergy testing by intradermal injection of various possible agents has low sensitivity and specificity, and in cases when management should be undertaken immediately such as during the perioperative period, such testing has no practical value (Armitage-Chan 2010). Tryptase and histamine blood levels are measured in humans in order to diagnose anaphylaxis (Sampson et al. 2006, Khan & Kemp 2011). This has limited application in companion animals possibly due to the limited time frame during which they should be measured. Therefore, they are impractical in emergency cases, as long as there is no evidence to support their usefulness in companion animals (Sampson et al. 2006, Schwartz 2006, Quantz et al. 2009, Mi et al. 2014).
From the above, diagnosis is based on history and clinical findings. The use of other diagnostic tools like identification of biomarkers or skin sensitivity test is rarely performed due to low sensitivity or low specificity to diagnose or predict an anaphylactic reaction.
Treatment
Anaphylaxis is a medical emergency (Simons 2009, Armitage-Chan 2010, Dowling 2014). Immediate and aggressive treatment strategies must be taken as cases that may not initially seem life-threatening could be fatal within minutes (Sampsons et al. 2006, Shmuel & Cortes 2013, Dowling 2014). Treatment options (Table 2) for anaphylaxis are based on the experience of the clinician and depend on the symptoms and clinician’s preferences. Usually the treatment begins with removal or discontinuation of the suspected agent (Shmuel & Cortes 2013). However, when a dog or a cat is presented with anaphylactic shock that may lead in cardiorespiratory collapse the RECOVER guidelines can be followed including rapid evaluation of the airway, breathing and circulation in order to rule out cardiopulmonary arrest (Sampson et al. 2006, Fletcher et al. 2012, Shmuel & Cortes 2013).
The drug of choice is adrenaline. Adrenaline is naturally produced by the adrenal medulla and is secreted in life-threatening situations from the adrenal glands (Mink et al. 1998, Bautista et al. 2002, Mink et al. 2004, Mathews 2006, Sampson et al. 2006, Kemp et al. 2008, Simons 2009, Simons et al. 2011, Fletcher et al. 2012, Dowling 2014). As a medication during management of anaphylaxis, adrenaline is mainly used for its effect on the cardiovascular system and smooth muscle cells (Shmuel & Cortes 2013) by activating alpha- and beta receptors (Gu et al. 1999). Its effect on the alpha-adrenergic receptors results in vasoconstriction, leading to increase in peripheral vascular resistance, increase in systemic arterial pressure and reduction of airway oedema (Gu et al. 1999). The activation of beta-adrenergic receptors results in positive inotropic and chronotropic effect (via beta-1-adrenergic receptors) on the myocardium, resulting in increase in cardiac output (Gu et al. 1999, Kemp et al. 2008). Other than managing cardiovascular signs, adrenaline results in bronchodilation, suppression of the release of inflammatory mediators and reduction of urticarial (via beta-2-adrenergic receptors) (Gu et al. 1999, Kemp et al. 2008, Shmuel & Cortes 2013). Side effects reported following adrenaline injections include pale mucous membranes and nervousness, which are usually mild and observed within minutes of administration. More severe side effects include myocardial ischemia, hypertensive crisis, ventricular arrhythmias and pulmonary oedema. However, these are usually attributed to overdose (Shmuel & Cortes 2013).
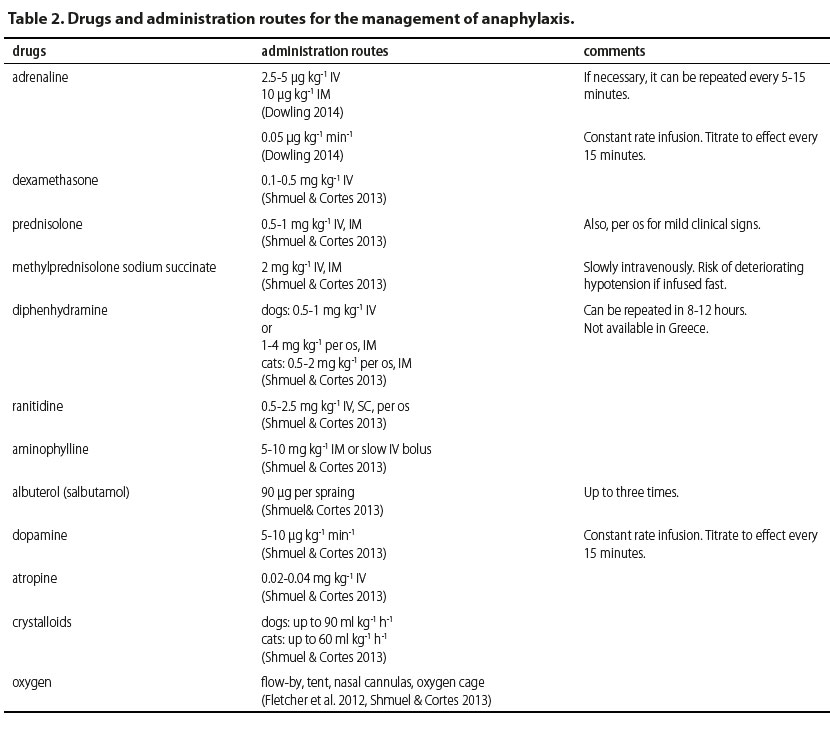
Recommended doses prior to circulatory collapse are at 2.5-5 μg kg-1 as an intravenous (IV) injection from a solution of 1/1000 (1 mg ml-1) or 10 μg kg-1 by intramuscular (IM) injection (Dowling 2014). If necessary, the injection can be repeated every 5-15 minutes (Shmuel & Cortes 2013). Adrenaline can also be injected by subcutaneous (SC) route (Mink et al. 1998, Gu et al. 1999, Mink et al. 2004, Sampson et al. 2006, Dowling 2014), however it should be avoided considering that absorption is delayed. Furthermore, low levels of adrenaline in tissues may lead to vasodilation and stimulate the secretion of inflammatory mediators (Mink et al. 2004, Dowling 2014). Mink et al (2004) showed that mean arterial pressure, pulse volume, cardiac output and cardiac work were increased in constant rate infusions compared to bolus injections. Therefore, in cases admitted in a state of shock, the recommended administration is by constant rate infusion, at a rate of 0.05 μg kg-1 min-1 with the dose titrated to clinical response (Mink et al. 1998, Mink et al. 2004, Dowling 2014). However, a different report showed that in animals with manifestations of anaphylaxis in which shock has been established and release of inflammatory mediators has already occurred, IV, IM or SC infusions are ineffective in reversing cardiovascular collapse (Mink et al. 1998, Bautista et al. 2002, Mink et al. 2004).
The second level of management includes other drugs. However, their role in managing anaphylaxis has not been substantiated. These include antihistamines, corticosteroids, bronchodilators, vasopressors, and anticholinergics (Lautt & Legare 1987, Silverman et al. 1988, Armitage-Chan 2010, Choo et al. 2010, Dowling 2014).
Antihistamine agents are mainly used to manage pruritus, urticaria, and rhinitis, symptoms originating from the skin and mucosae and not for severe systemic manifestations of anaphylaxis (Simons et al. 2011). In general, first-generation (Η1) or second-generation (Η2) antihistamines are used. First-generation Η1-antihistamines include diphenhydramine, chlorpheniramine, cyproheptadine and hydroxyzine (Silverman et al. 1988, Viscasillas et al. 2011, Shmuel & Cortes 2013, Dowling 2014). They are considered to be less safe compared to second-generation antihistamine drugs because they cross the blood-brain barrier and may cause clinical signs from the central nervous system, such as fatigue, lethargy and cognitive dysfunction, as well as clinical signs from the digestive tract, such as vomiting, diarrhoea or anorexia (Shmuel & Cortes 2013). Second-generation antihistamines, including loratadine, fexofenadine and cetirizine are considered to be safer; however, they are not available for parenteral use. Even though Η2-antihistamines are considered to be safer than H1, they are generally considered to be ineffective, at least as sole agents, in managing anaphylaxis except for limiting manifestations from the digestive tract (Shmuel & Cortes 2013, Dowling 2014). In general, there have been no published reports about antihistamines that may substantiate any benefit from using them in managing anaphylaxis in companion animals. However, diphenhydramine has been recommended at 0.5-1 mg kg-1 IV, whereas for IM or oral administration in dogs it is recommended at 1-4 mg kg-1 and for cats at 0.5-2 mg kg-1. Repeated doses can be administered after 8-12 hours (Shmuel & Cortes 2013).
Corticosteroids are widely used in allergic reactions, which have led to their integration in managing anaphylaxis to the point that they are sometimes used erroneously as first choice drugs, considering that there are no publications to substantiate this (Sampson et al. 2006, Choo et al. 2010, Girard & Leece 2010). They prevent arachidonic acid and prostanoid formation, therefore suppressing the clinical signs of delayed reaction; however, this has not yet been substantiated (Sampson et al. 2006, Dowling 2014). Morover, corticosteroids have a delayed onset of action, about 4-6 hours, regardless of administration route, therefore they are ineffective in the initial stages of anaphylaxis which may be life threatening (Shmuel & Cortes 2013, Dowling 2014). In general, they are administered as a single bolus (Choo et al. 2010). Furthermore, they are a drug category which has been reported to potentially cause anaphylaxis. There is even a case report of a death in a dog attributed to anaphylactoid reaction after administration of dexamethasone (Schaer et al. 2005). Dexamethasone is administered at 0.1-0.5 mg kg-1 IV, prednisolone at 10-25 mg kg-1 IV and methylprednisolone at 30 mg kg-1 IV. Prednisolone can be given orally at 0.5-1 mg kg-1 in order to manage very mild clinical signs (Shmuel & Cortes 2013, Dowling 2014).
In some cases, in either animals or people who have previously undergone an anaphylactic episode, preventive use of antihistamines or corticosteroids is recommended (Armitage-Chan 2010, Choo et al. 2010, Girard & Leece 2010). However, this does not prevent the advent of anaphylaxis but only blunts the natural systemic response (Shmuel & Cortes 2013). Thus, despite widespread use antihistamines and/or corticosteroids for prophylactic pre-treatment in humans, their help is not evidenced in the preoperative period (Kroigaard et al. 2007, Liccardi et al. 2008).
Bronchodilators such as albuterol and aminophylline can also be used, mostly in controlling respiratory symptoms. Albuterol is a beta-2-adrenergic agonist and usually administered in spray form through the respiratory tract (Simons et al. 2011). It can be valuable when clinical signs from the lower respiratory tract are present, but it is ineffective in case of laryngeal or tracheal oedema. Aminophylline can also be given considering that it increases endogenous production of adrenaline and results in lower airway smooth muscle cell relaxation (Shmuel & Cortes 2013). Bronchodilators are not a first line treatment for anaphylaxis and cannot replace adrenaline. They can be effective only in the early stages of respiratory signs (Sampson et al. 2006, Shmuel & Cortes 2013, Dowling 2014). Recommended doses are for aminophylline 5-10 mg kg-1 IM or slow IV bolus, and for albuterole 90 μg per spray, up to three times in total (Shmuel & Cortes 2013).
Other treatment options include the administration of vasopressors like dopamine, noradrenaline and anti-diuretic hormone in order to increase systemic arterial pressure in refractory hypotension (Lautt & Legare 1987, Shmuel & Cortes 2013, Dowling 2014). Dopamine has a dose-related effect, with an initial increase in myocardial contractility followed by vasoconstriction in higher doses due to beta-1- and alpha-1-adrenergic receptor activation. Noradrenaline can also be used for its vasopressor properties. Although anti-diuretic hormone has not been studied in companion animals, there have been case reports with severe hypotension which did not respond to dopamine but responded to anti-diuretic hormone (Kill et al. 2004, Silverstein et al. 2007, Scroggin & Quandt 2009). Atropine is recommended in patients with refractory bradycardia (Dowling 2014).
Intravenous fluids are the mainstay of treatment in the management of anaphylaxis (Shmuel & Cortes 2013, Dowling 2014). Arterial dilation leads to extravasation of large volumes of water from arterioles and pooling of blood, resulting in dramatic decreases of circulating blood volume (Brown 2005). Therefore, the aim is to increase intravascular volume, to increase systemic arterial pressure and to prevent the advent of shock. Intravenous fluids that may be used are isotonic crystalloids (up to 90 ml kg-1 in dogs, 60 ml kg-1 in cats, in total) or colloids (e.g. hetastarch) in IV boluses of 5 ml kg-1 per infusion, up to total daily dose of 20 ml kg-1 for rapid volume replacement (Shmuel & Cortes 2013, Dowling 2014).
Oxygen administration via flow-by, tent, nasal cannulas or oxygen cage is of utmost importance in such patients especially when respiratory manifestations are present (Armitage-Chan 2010, Shmuel & Cortes 2013, Dowling 2014).
Finally, animals with severe anaphylaxis, which are admitted in shock, should be closely monitored and if possible there should be constant monitoring with electrocardiography for the evaluation of heart rate and rhythm, measurement of systemic arterial pressure, assessment of oxygenation and ventilation, evaluation of end-tidal CO2 and urine production, due to the fact that such cases may manifest biphasic anaphylaxis, meaning that there may be initial improvement of clinical signs with subsequent relapse (Fletcher et al. 2012, Shmuel & Cortes 2013). Close monitoring is recommended for three days in cases of biphasic anaphylaxis, which has been reported in dogs up to 48 hours after the initial episode (Shmuel & Cortes 2013).
Conclusion
Perioperatively anaphylaxis is attributed to several drugs including anaesthetics and antibiotics. Agents which have been implicated for anaphylaxis should be avoided when feasible (Shmuel & Cortes 2013, Dowling 2014). In such cases, the only way of prevention is slow infusion if the intravenous route is necessary, especially for drugs with no reports on their relevant reports (Armitage-Chan 2010). There are still several unanswered questions regarding why some patients manifest mild clinical signs whereas to others anaphylaxis may be fatal, and why some cases will not recover despite timely aggressive management whereas in other cases clinical signs may improve even without treatment (Armitage-Chan 2010, Shmuel & Cortes 2013). A step towards to a better description of the phaenomenon perioperatively will be the recording and publication of any case of anaphylaxis
References
- Armitage-Chan E (2010) Anaphylaxis and anaesthesia. Vet Anaesth Analg 37, 306-310.
- Bautista E, Simons FE, Simons KJ et al. (2002) Epinephrine fails to hasten hemodynamic recovery in fully developed canine anaphylactic shock. Int Arch Allergy Immunol 128, 151-164.
- Berdoulay P, Schaer M, Starr J (2005) Serum sickness in a dog associated with antivenin therapy for snake bite caused by Crotalus adamanteus. J Vet Emerg Crit Care 15, 206-212.
- Blake MK, Carr BJ, Mauldin GE (2016) Hypersensitivity reactions associated with L-asparaginase administration in 142 dogs and 68 cats with lymphoid malignancies: 2007-2012. Can Vet J 57(2), 176- 182.
- Brown S (2005) Cardiovascular aspects of anaphylaxis: implications for treatment and diagnosis. Curr Opin Allergy Clin Immunol 5, 359-364.
- Burren VS, Manson KV (1986) Suspected anaphylaxis to thiopentone in a dog. Austral Vet J 63, 384-385.
- Carter JE, Chanoit G, Kata C (2011) Anaphylactoid reaction in a heratworm-infected dog undergoing lung lobectomy. J Am Vet Med Assoc 238, 1301-1304.
- Choo KJ, Simons FE, Sheikh A (2010) Glucocorticoids for the treatment of anaphylaxis: Cochrane systematic review. Allergy 65, 1205-1211.
- Descotes J, Payen C, Vial T (2007) Pseudo-allergic drug reactions with special reference to direct histamine release. Perspect Exp Clin Immunotoxicol 1, 41-50.
- Dowling P (2014) Anaphylaxis. In: Small Animal Critical Care Medicine. 2nd ed. Silverstein D, Hopper K. WB Saunders: St Louis, Missouri, pp. 807-810.
- Fletcher DJ, Boller M, Brainard BM et al. (2012) RECOVER evidence and knowledge gap analysis on veterinary CPR. Part 7: Clinical guidelines.J Vet Emerg Crit Care 22(s1), S102-S131.
- Francis H, Martin L, Haldorson G et al. (2007) Adverse reactions suggestive of type III hypersensitivity in six healthy dogs given human albumin. J Am Vet Med Assoc 230, 873-879.
- Girard NM, Leece EA (2010) Suspected anaphylactoid reaction following intravenous administration of a gadolinium- based contrast agent in three dogs undergoing magnetic resonance imaging. Vet Anaesth Analg 37, 352-356.
- Guedes AG, Rude EP, Rider MA (2006) Evaluation of histamine release during constant rate infusion of morphine in dogs. Vet Anaesth Analg 33, 28-35.
- Gu X, Simons FE, Simons KJ (1999) Epinephrine absorption after different routes of administration in an animal model. Biopharm Drug Dispos 20, 401-405.
- Haworth M, McEwen M, Dixon B et al. (2019) Anaphylaxis associated with intravenous administration of alphaxalone in a dog. Aust Vet J 97, 197-201.
- Hepner DL, Castells M (2003) Anaphylaxis During the Perioperative Period. Anesth Analg 97, 1381-1395.
- Hume-Smith KM, Groth AD, Rishniw M et al. (2011) Anaphylactic events observed within 4 h of ocular application of an antibioticcontaining ophthalmic preparation: 61 cats (1993-2010). J Feline Med Surg 13, 744-751.
- Jackson HA, Jackson MW, Coblentz L et al. (2003) Evaluation of the clinical and allergen specific serum immunoglobulin E responses to oral challenge with cornstarch, corn, soy and a soy hydrolysate diet in dogs with spontaneous food allergy. Vet Dermatol 14, 181–187.
- Jones RS (1992) Muscle relaxants in canine anaesthesia 1: History and the drugs. J Small Anim Pract 33, 371-375.
- Kemp SF, Lockey RF, Simons FE (2008) Epinephrine: the drug of choice for anaphylaxis: a statement of the World Allergy Organization. WHO Journal S2, S18-S26.
- Khan BQ, Kemp SF (2011) Pathophysiology of anaphylaxis. Curr Opin Allergy Clin Immunol 11, 319–325.
- Kill C, Wranze E, Wulf H (2004) Successful treatment of severe anaphylactic shock with vasopressin. Int Arch Allergy Immunol 134, 260–261.
- Kroigaard M, Garvey LH, Gillberg I et al. (2007) Scandinavian clinical practice guidelines on the diagnosis, management and follow-up of anaphylaxis during anaesthesia. Acta Anaesthesiol Scand 51(6), 655–670.
- Küls N, Rocchi A, Larenza PM (2016) Suspected anaphylaxis after intravenous injection of rocuronium in a dog. Vet Rec Case Rep 4, 1-5.
- Lautt WW, Legare DJ (1987) Effect of histamine, norepinephrine, and nerves on vascular pressures in dog liver. Am J Physiol 252, G472–G478.
- Liccardi G, Lobetalo G, Di Florio E et al. (2008) Strategies for the prevention of asthmatic, anaphylactic and anaphylactoid reactions during the administration of anesthetics and/or contrast media. J Investig Allergol Clin Immunol 18, 1–11.
- Litster A, Atwell R (2006) Physiological and haematological findings and clinical observations in a model of acute systemic anaphylaxis in Dirofilaria immitis sensitized cats. Aust Vet J 84, 151–157.
- Mason TA (1976) Anaphylactic response to thiopentone in a dog. Vet Rec 98, 136.
- Mathews KA (2006). Anaphylactic and anaphylactoid reactions. In: Veterinary Emergency and Critical Care Manual. 2nd ed. Mathews KA ed Life Learn, Ontario, Canada, pp. 615-618.
- Mertes PM, Laxenaire MC (2004) Anaphylactic and anaphylactoid reactions occurring during anaesthesia in France. Seventh epidemiologic survey (January 2001- December 2002). Ann Fr Anesth Reanim 23, 1133–1134.
- Meyer EK (1997) Rare, idiosyncratic reaction to acepromazinein dogs. J Am Vet Med Assoc 210, 1114–1115.
- Mi Y-N, Ping N-N, Xiao X et al. (2014). The Severe Adverse Reaction to Vitamin K1 Injection Is Anaphylactoid Reaction but Not Anaphylaxis. PLOS ONE 9, 1-10.
- Mink SN, Bands C, Becker A et al. (1998) Effect of bolus epinephrine on systemic hemodynamics in canine anaphylactic shock. Cardiovasc Res 40, 546–556.
- Mink SN, Simons FE, Simons KJ et al. (2004) Constant infusion of epinephrine, but not bolus treatment, improves haemodynamic recovery in anaphylactic shock in dogs. Clin Exp Allergy 34, 1776–1783.
- Moore GE, DeSantis Kerr AC, Guptiill LF et al. (2007) Adverse events after vaccine administration in cats: 2560 cats (2002-2005). J Am Vet Med Assoc 231, 94-100.
- Moore GE, HogenEsch H (2010) Adverse vaccinal events in dogs and cats. Vet Clin N Am Small Anim Pract 40, 393–407
- Peavy RD, Metcalfe DD (2008) Understanding the mechanisms of anaphylaxis. Curr Opin Allergy Clin Immunol 8, 310–315.
- Pollard RE, Pascoe PJ (2008) Severe reaction to intravenous administration of an ionic iodinated contrast agent in two anesthetized dogs. J Am Vet Med Assoc 233, 274–278.
- Portier P, Richet C (1902) De l’action anaphylactique de certains venins. C R Séances Soc Biol 54,170
- Quantz J, Miles M, Reed A et al. (2009) Elevation of alanine transaminase and gallbladder wall abnormalities as biomarkers of anaphylaxis in canine hypersensitivity patients. J Vet Emerg Crit Care 19, 536–544.
- Raptopoulos D, Papazoglou L, Galatos A (1993) Suspected adverse reaction to xylazine-ketamine anesthesia in a cat. Fel Pract 21, 27–29.
- Richardson PD, Withrington PG (1978) Responses of the simultaneously-perfused hepatic arterial and portal venous vascular beds of the dog to histamine and 5-hydroxytryptamine. Br J Pharmacol 64, 581–588.
- Rostaher A, Hofer-Inteeworn N, Kümmerle-Fraune C, Fischer NM, Favrot, C. (2017). Triggers, risk factors and clinico-pathological features of urticaria in dogs - a prospective observational study of 24 cases.Vet Dermatol 28(1), 39–46.
- Sampson HA, Munoz-Furlong A, Campbell RL et al. (2006) Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium. J Allergy Clin Immunol 117, 391–397.
- Schachter M (1952) The release of histamine by pethidine, atropine, quinine, and other drugs. Brit J Pharmacol 7, 646–653.
- Schaer M, Ginn PE, Hanel RM (2005) A case of fatal anaphylaxis in a dog associated with a dexamethasone suppression test. J Vet Emerg Crit Care 15, 213–216.
- Scroggin RD, Quandt J (2009) The use of vasopressin for treating vasodilatory shock and cardiopulmonary arrest. J Vet Emerg Crit Care 19, 145–157.
- Schwartz LB (2006) Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin N Am 26, 451–463.
- Shmuel D, Cortes Y (2013) Anaphylaxis in dogs and cats. J Vet Emerg Crit Care 23, 377-394.
- Silverman HJ, Taylor WR, Smith PL et al. (1988) Effects of antihistamines on the cardiopulmonary changes due to canine anaphylaxis. J Appl Physiol 64, 210–217.
- Silverstein DC, Waddell LS, Drobatz KJ et al. (2007) Vasopressin therapy in dogs with dopamine resistant hypotension and vasodilatory shock. J Vet Emerg Crit Care 17, 399–408.
- Simons FE, (2009) Anaphylaxis: Recent advances in assessment and treatment. J Allergy Clin Immunol 124, 625–636.
- Simons FE, Ardusso LR, Bilo MB et al. (2011) World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J 4, 13-37.
- Threlfall A, Viscasillas J, Volk A (2012). Localised cutaneous reaction after epidural administration of preservative free morphine and ropivacaine? Vet Anaesth Analg, 39(5), 559–560. #Viscasillas J, Seymour C, Knudsen T, Levien A, Volk A (2011). Cutaneous reaction after intravenous administration of medetomidine? Vet Anaesth Analg, 38(4), 413–414.
Corresponding author:
Vasileia Angelou
e-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.



