Panagiota Karamichali DVM, MSc, Kiriaki Giamoustari DVM, Anastasia Komnenou DVM, PhD
Companion Animal Clinic, School of Veterinary Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
MeSH keywords:
analgesia, fluid therapy, intraoperative monitoring, rabbits, Rodentia
Abstract
The article describes the appropriate handling during the pre-anaesthetic physical examination, the administration of fluids, the monitoring of vital functions during surgery and finally the evaluation and management of perioperative pain in rabbits and domestics rodents.
Introduction
The popularity of rabbits and domestic rodents (mouse, rat, guinea pig, gerbil, hamster) is increasing, as well as the number of surgical procedures they are subjected to. A big amount of information comes from their use as laboratory animals. However, the perioperative mortality rate (1.39%) is 6-9 times higher than the corresponding rates in dogs (0.17%) and cats (0.24%) (Brodbelt 2009). Consequently, the perioperative care of these animals is a challenge for the veterinary clinician. The aim of the present review is to present ways of obtaining best results, maximum safety and minimal stress for these animal species in routine clinical practice. In this context, the appropriate handling during the preanaesthetic clinical examination, fluid therapy, the monitoring of vital functions and the management of postoperative pain will be analysed.
Physical examination
Rabbits and domestic rodents are vulnerable to stress: carrier cage, environmental temperature, handling by the examiner, loud noises, light, pain etc. may cause increase in levels of cortisol, catecholamines, and thyroxine, as well as decrease in insulin levels. Perioperative stress can extensively affect the postoperative patient status, including standing/movement, food consumption, self-grooming, and general clinical status (Canon et al. 2011, Gaskil & Garner, 2017).
Handling by the veterinarian during physical examination should be specific, with steady movements and in a quiet environment isolated from other animals in the veterinary clinic. In particular, for each species the following are suggested:
Rabbit
In general, rabbits are well behaved animals. In cases when an aggressive rabbit is examined, the main danger originates from the claws which may cause deep lacerations and the incisors, which may cause deep injuries. Handling should be done in a mild and steady manner. Particular attention should be given in restraining the hind limbs, considering that a forceful and sudden kick may result in spinal fractures. Moreover, in some cases severe stress during examination can result in cardiac arrest. It is therefore necessary that short and safe restraining are provided by the examining clinician (Flecknell 2006).
Restraining can be done with one hand on the scruff of the neck and the other controlling the hind limbs by positioning them vertically. Alternatively, one hand can be placed under the chest, with the thumb and first two fingers controlling the anterior limbs, whereas the other is placed under the hindquarters so that the spine can be supported (Figure 1A). When the rabbit is carried it must be held close to the examiner’s body, so that the body of the animal rests on one arm and the other hand controls the back and scruff of the neck (Figure 1B). One more restraint technique is holding the rabbit in a supine position, so that the back lies on the examining table and one of the examiner’s hand is on the chest of the animal. This is a behavioural pattern of immobility position, which the animals exhibit in nature when they pretend that they are dead to a predator. However, this position is highly stressful for the animal, therefore painful manipulations during handling in this manner should be avoided. In no instance should rabbits ever be restrained or raised by the ears alone (Meredith & Crossley 2002).
Significant stress reduction can be accomplished if the eyes are covered by hand or the head is wrapped in a towel (Figure 1C). In this manner the examination of the oral cavity and ears can be accomplished. Particular attention must be given during handling in a towel due to a risk for hyperthermia. Therefore, environmental temperature should not surpass 23-25ºC (Flecknell 2006).
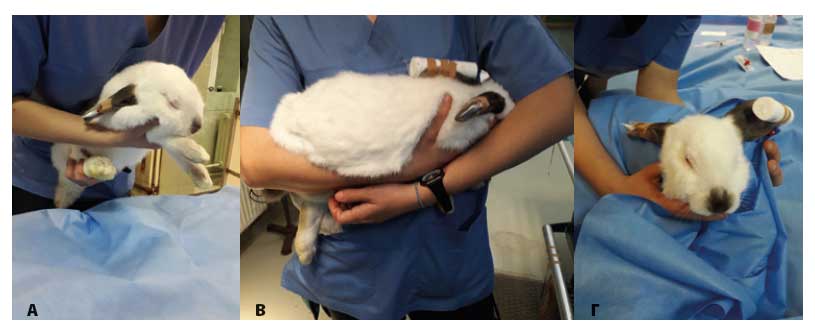
Figure 1. Handling of a rabbit during physical examination. A: One hand of the examiner is placed under the chest, with the thumb and first two digits controlling the front limbs, whereas the other hand is placed under the hind limbs so that the spine is supported. B: The body of the animal is supported on the forearm of the examiner with the head between the examiner’s body and the arm. C: The body of the animal is wrapped in a towel in order to minimise stress (author’s PK personal files).
Mouse
Handling in the mouse must be restricted to a minimum, considering that this animal usually bites an unknown handler, especially in an unfamiliar environment. Restraining is possible by grasping the mouse by the tail base and transporting it to a non-slippery surface, where it will instinctively move toward the opposite direction. Next, the tail is restrained by one hand and the scruff of the neck is grasped with the thumb and index finger of the other. It is useful to grasp sufficient skin surface, so that the animal cannot turn its head to bite, but also not so much that respiration is impeded. The examiner can then raise the mouse by holding the tail (Orr 2002).
Thermoregulation in this animal species occurs through the blood vessels of the tail just like in rats (Owens et al. 2002). In cases of reduction in core temperature, there is vasoconstriction of the tail blood vessels and in this way up to 17% of the total body heat can be maintained (Monson and Oyama 1984). Therefore, continuously keeping these animals warm by artificial means (blankets, heat lamps) is necessary and ideal surgical room temperature should be at 26-28ºC (Orr 2002).
Rat
In contrast with mice, rats rarely bite, especially if they have grown accustomed to human contact from a young age. Just like mice, rats are nocturnal, therefore in this species it should also be ensured that they are alert during physical examination (Tranquilli et al. 2013). They can be restrained with the thumb and the rest of the fingers of one hand enclosing the thorax at the level of the shoulders, whereas the other hand is placed under the hind limbs, thus supporting the body weight. In painful procedures the examiner’s thumb is placed under the lower jaw, so that the animal cannot bite. In cases of aggressive animals, they may be lifted by seizing the tail base. The carrying and handling of rats by the scruff of the neck is highly stressful, in contrast with mice, therefore it should be avoided (Orr 2002).
Hamster
Handling of the hamster can be challenging, however if it is adequately restrained, injuries can be avoided. When the hamster has been relatively accustomed to people, it can be carried and contained in the palms of both hands. In more aggressive animals, after they have been transported onto non-slippery surfaces, restraining is accomplished by mild but decisive motions, with the thumb and fingers of one hand seizing hold of the scruff of the neck. Next, the skin is moved on this spot toward the head, in order to confirm that sufficient skin has been grasped. In this manner injury to the examiner can be avoided, and at the same time the skin around the neck of the animal is not stretched. In cases of intense and poor handling of the skin around the neck proptosis of the eyes is often occur. When the animal is severely aggressive, the use of a small glass container or a barred cage with a cover can be valuable for examination and transport. Ideal environmental temperature for these animals is at 20- 25ºC (Harkness and Wagner 1995, Goodman 2002).
Gerbil
In the gerbil, in cases of poor handling limb fractures are common. It should therefore be examined on a table or flat surface, so that if it jumps out of fear and falls it will not suffer injuries. It is not an aggressive animal, it rarely bites, whereas handling by the tail is forbidden, because the skin may easily be degloved and the underlying bone be exposed. Simple transport can be done easily in the palms of both hands (just like hamsters). Full restraint is accomplished by either taking hold of the scruff of the neck with the thumb and fingers of one hand and supporting the weight of the animal with the other hand or supporting the body in both hands and the thumbs placed under the lower jaw. Ideal environmental temperature is at 20-25ºC (Cruz and Junquera 1993, Wurbel 2001, Kebble E 2002).
Guinea pig
The guinea pig is a mildly tempered animal with calm demeanour, however it can be easily stressed when placed in a non-familiar environment. Avoidance of exposure to intense light and sounds assists the examination and handling. It does not bite, however it may run fast in order to avoid being restrained. Handling is accomplished by grasping the guinea pig quickly and steadily around the shoulders with one hand and supporting the weight of the animal under the hind limbs with the other hand. The guinea pig has a large abdominal cavity, but thin bones and a fragile spine. Therefore, throughout the examination it is important to support the body weight in the examiner’s hand, in order to avoid injury. Particular care during handling should be given in not exerting pressure on the abdominal cavity, because there is a risk of liver rupture. Ideal environmental temperature is at 18-23ºC (Flecknell 2002, Wagner 2014).
Chinchilla
The chinchilla is easy to handle and rarely bites. For physical examination a quiet environment is required because it gets easily stressed. It moves very fast and it is capable jumper, therefore it is best to restrain it directly inside the cage and then carry it onto the examination table. Approaching the animal must be done in a calm manner, holding the thorax with one hand and supporting the body weight with the other. Alternatively, it can be restrained by the tail base and the body supported as previously mentioned. Even though the fur is thick, it is not firmly attached to the skin and can be easily epilated, a characteristic used as a defensive mechanism in the wild. Therefore, restraint by the scruff of the neck should be avoided, because it is too easy to remove the fur on that spot. Furthermore, when some chinchillas are stressed, they turn their hindquarters and can urinate on their handler with extraordinary accuracy. Finally, they have no sweat glands and are prone to heat stroke when environmental temperature is above 28ºC (Alworth 2012).
Venous access and fluid administration
In Table 1, administration rates and doses of commonly used fluids in rabbits and domestic rodents are shown.
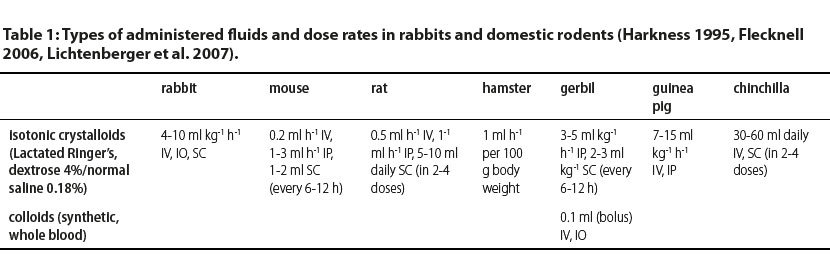
IV: intravenously, IO: intraosseously, IP: intraperitonealy, SC: subcutaneously
In all surgical procedures, except for certain minor ones, warm isotonic crystalloid administration is recommended, ideally through an intravenous catheter. In rabbits, for fluid administration the marginal auricular and cephalic vein are preferred (22G catheter in rabbits usually larger than 3 kg, 24-26 G catheter in smaller rabbits) (Lichtenberger 2007). Alternative routes include subcutaneous and intraperitoneal, but not for fluids containing dextrose. Peripheral vein catheterisation can be facilitated by topical application of an anaesthetic cream (e.g. lidocaine-prilocaine, EMLA cream™). Effective local anaesthesia can be ensured after topical application of the cream on the skin of the catheterisation site, 15 minutes up to one hour prior to venous catheterisation and bandaging with plastic wrap and self-adherent patch (Flecknell 1990, Erkert & MacAllister 2005, Keating et al. 2012). A similar anaesthetic effect has been reported with local application of 1 ml of EMLA cream™, after shaving of the skin over the catheterisation site without bandaging, for 10 minutes prior to catheterisation (Kazakos et al. 2010). Even though there have been no reports of toxicosis (e.g. methaemoglobinaemia) in small mammals after the application of EMLA cream™, it is recommended to follow application guidelines (amount, size of application surface etc.).
In rats the lateral vein of the tail is used. In young animals or animals with cardiovascular collapse the intraosseous route is valuable, in which the needle can remain for up to 72 hours without flushing (if the fluid administration is not continuous, catheter flushing is required twice daily). From the long bones, femur and tibia are most commonly used in routine practice for intraosseous fluid administration (Lennox 2008, Edis 2016). Intraosseous catheterisation is mostly used for short term fluid administration, until an intravenous catheter can be introduced. The intraosseous catheter may be uncomfortable and may lead to osteomyelitis due to non-aseptic conditions of placement or being maintained for long time periods. In septic patients and patients with metabolic disorders of the bones it is contraindicated (Quesenberry & Carpenter 2012).
Because of the small size of these animals the correct calculation of the total amount of administered fluids is of key importance, in order to prevent complications from fluid overload. For example, subcutaneous and intramuscular injection of high volumes of fluids has been reported to cause pain, local irritation, and necrosis of muscle tissues and self-mutilation of the limb (Smiler et al. 1990). Complications from erroneously excessive administration of intravenous fluids included shock, respiratory distress, haemodynamic instability or even death (Morton et al. 1997, Morton et al. 2001).
Monitoring
The monitoring of vital signs is essential to begins from the preanaesthetic physical examination, lasting until the recovery from anaesthesia. Most surgical procedures can be performed when the withdrawal reflex is completely or almost abolished (Bannon & Malberg 2007, Deuis 2017). In guinea pigs the auricular reflex can be used as an alternative. Ocular reflexes are not often used because they are usually maintained to some degree intraoperatively. Their absence indicates deep anaesthesia (Quesenberry & Carpenter 2012).
The small size of rabbits and domestic rodents may cause problems in the function of some monitoring devices. Small electrical potential differences developed in the myocardium may be poorly detected by some monitors. Moreover, these species have high heart rates which can surpass the maximum sensitivity of pulse oximeters. Small tidal volumes and week pulse limit the use of capnographs and blood pressure monitors, respectively. In rabbits and large rodents, respiratory rate monitors with a thermistor may be adequately functional, but they usually fail to detect expiration in smaller animals.
Despite limitations, monitoring can be accomplished. Special equipment (e.g. capnographs) is commercially available internationally for small mammals. Pulse oximeters are commercially available with the ability of tracing high frequency heart rates (scanning speed 100-200 mm sec-1, instead of the standard 25 mm sec-1) and the sensors can be placed on the paws or mouth of larger species (more than 200 g). The needles on the electrodes of cardiographs are ideal for small rodents, since they provide best conductivity without the use of gel. Alternatively, metallic clasps attached to hypodermic needles or with cotton dipped in salt solution can be used (Flecknell 2006). Measuring arterial pressure in rabbits, when their size permits, can be done either directly (after introduction of an arterial catheter in the auricular artery) or indirectly with the oscillometric or the Doppler technique. Studies have shown that non-invasive blood pressure monitoring is reliable both in normal and hypotensive rabbits (Ypsilantis et al. 2005, Harvey et al. 2012, Bellini et al. 2018).
The respiratory rate during anaesthesia is 30- 60 min-1 in rabbits and 50-100 min-1 in rodents. Reduction of the respiratory rate more than 50% must be treated as an emergency (Flecknell 2006, Quesenberry & Carpenter 2012). It is worthy of note that cyanosis of the tongue in rabbits with a laryngeal mask may be a result of pressure exerted by the device, due to the small size of the oral cavity and may not indicate a systemic disorder (Kazakos et al. 2007).
In order to measure temperature, it is important to use a thermometer which measures values of less than 35ºC, as long as temperature drop may be significant in these animals (Flecknell 2006). Ideally, fluids are administered intravenously or intraosseously, during the perioperative and intraoperative period. If either option is unavailable, fluids are administered subcutaneously or intraperitoneally (absorption is considerably slower in this case).
Analgesia
Pain assessment
Assessment of postoperative pain in rabbits and domestic rodents is more difficult than in dogs and cats. Even though pain signs are distinct, veterinary clinicians are usually not accustomed to the normal behavioural patterns of these species. These animals, being prey, tend to conceal painful conditions and maintain normal patterns of behaviour, in order to avoid capture. The human presence can cause full immobility, without apparent indications of pain. The usual clinical signs of pain include reduced appetite, change in mobility and social activity, changes in posture and gait and licking or chewing of the painful body area. Anorexia is a critical indication of pain and can follow reduced gastrointestinal motility (Wenger 2012).
In rabbits and mice (and in some other laboratory animals) a graded scale for measuring postoperative pain has been described, known as “rabbit grimace scale” and “mouse grimace scale”, respectively (Langford et al. 2010, Keating et al. 2012). This scale is based on facial indicators, such as the palpebral fissure length, the cheekbone shape, the muzzle shape, the fur of the skull and the shape and position of the ears. According to the sum of grading, it is decided whether rescue analgesia should be administered (Hampshire & Robertson 2015). Limitations of this scale are that it is applied to certain breeds and animal species, specific surgical procedures and it has not found extensive application outside the laboratory setting (Miller & Leach 2015).
Pain management
Identification of pain as early as possible is vital for adequate management prior to the development of undesirable sequelae (Kohn et al. 2007). In rabbits, anorexia exceeding 1-2 days can rapidly lead to potentially life-threatening gastrointestinal stasis (Wenger 2012). Pain contributes to immunosuppression and the consequent risk of infection should be taken into consideration in these animals, including rabbits, in which subclinical respiratory infections are common. An effective way of pain management is multi-modal analgesia (Wenger 2012). An effective multi-modal plan commonly includes combinations of different categories of analgesic drugs, such as opioids, non-steroidal anti-inflammatory drugs (unless contraindicated), local anaesthetics, α-2 agonists, ketamine, gabapentin, etc. (Benato et al. 2019). Moreover, non-pharmacological support with acupuncture, cold packs, minimally invasive surgical procedure, careful handling, low frequency laser, etc. May be applied (Berry 2015). In Table 2, doses of the most commonly used analgesics in rabbits and domestic rodents are presented.
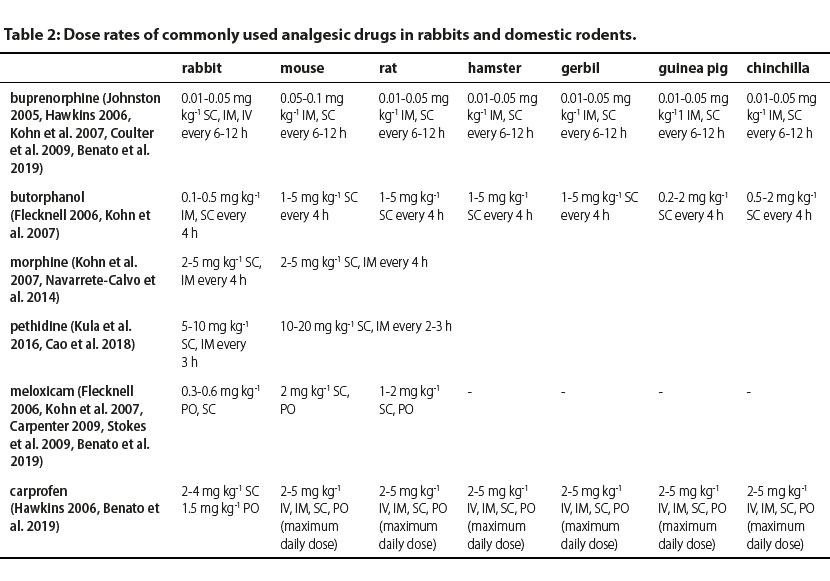
IV: intravenously, IM: intramuscularly, SC: subcutaneously, PO: per os
Opioids
Harmful sequelae of pain on gastrointestinal motility can be managed with the appropriate administration of opioids in rabbits and domestic rodents, especially in species in which non-steroidal anti-inflammatory drugs are contraindicated (Swisher et al. 2015). The use of fentanyl in high doses necessitates intubation and positive pressure ventilation on standby, especially in combination with sedatives (Barter 2011). Fentanyl can be administered in combination with a sedative that suppresses some of its undesirable side effects, such as vomiting and hyperactivity. When used as a sole agent, it results in mild skeletal muscle relaxation, and is therefore inappropriate for any procedure other than a short surgery. Fentanyl dose can be reduced by 50-70%, when it is administered with a benzodiazepine, and with this combination sufficient muscle relaxation is obtained. When morphine is used in rabbits, it is most effective in managing mild to severe pain, but it is rarely used due to its side effects, including sedation, respiratory depression and reduced gastrointestinal motility (Barter 2011). In rats and mice, butorphanol is used to manage mild pain of short duration and it is administered every 1-2 hours (Gades et al. 2000), whereas in rabbits it is recommended for mild to moderate pain of short (2-3 hours) duration (Barter 2011). The use of tramadol, although it is widely available in the Greece, in these animals is contraindicated. Especially in rabbits its administration either orally or intravenously has failed to show positive analgesic results (Souza et al. 2008, Egger et al. 2009).
Non-steroidal anti-inflammatory drugs (NSAIDs)
The most commonly used NSAIDs in rabbits and domestic rodents are meloxicam, carprofen and ketoprofen (Barter 2011). In practice, meloxicam is most commonly used (Bourque et al. 2010; Wenger 2012). After oral administration in rabbits, meloxicam reaches maximum concentrations in plasma and is metabolised faster than in dogs, rats and humans (Turner et al. 2006). In rabbits, doses higher than 0.3 mg kg-1 24h-1 may be required in order to maintain maximum concentrations in plasma for up to 24 hours. A dose of 1.5 mg kg-1 SC or PO is well tolerated for up to 5 days (Carpenter et al. 2009, Fredholm et al. 2013). The honey taste of oral meloxicam renders this drug palatable for animals and it is appropriate for pain management at home (Leach et al. 2009, Wegner 2012). However, oral doses of meloxicam may not be sufficient for adequate management of pain after intra-abdominal surgery (e.g. ovariohysterectomy). Even though meloxicam doses of 0.2 mg kg-1 have anti-inflammatory properties in rats, it is reported in the literature that higher doses are necessary, up to 1-2 mg kg-1 for the management of postoperative pain (Roughan et al. 2003).
Topical anaesthesia
Lidocaine and bupivacaine are the most commonly used local anaesthetics in topical infiltration and intra-articularly. Lidocaine has a quick onset of action but short duration, whereas bupivacaine has a slow onset but longer duration. It is recommended for local anaesthetics to be diluted 1:2 and 1:4 in order to prevent overdose. Local infiltration of tissues in surgical or non-surgical wounds is a simple and cheap method of analgesia for surgical procedures. Adding adrenaline to the solution intended for tissue infiltration can prolong the duration of action of local anaesthetics (Wegner 2012, Kluge et al. 2017).
Regional analgesia
Epidural analgesia (0.1-0.2 mL kg-1) and femoral and sciatic nerve block have been described in guinea pigs, rats and rabbits, for procedures on the hind limbs. In rabbits and rats, epidural analgesia is used for intraoperative as well as postoperative analgesia. In cases when prolonged analgesia is required, an epidural catheter can be placed for continuous or repeated bolus administration (van den Hoogen et al. 1981, Malinovsky et al. 1997, Nishiyama 1998, Dollo et al. 2004, Johnston 2005, Lichtenberger et al. 2007, Wenger 2012, Aguiar et al. 2014).
Epilogue
In conclusion, rabbits and domestic rodents have increased needs regarding correct perioperative management. Attention to stress reduction as early as from the preanaesthetic evaluation, to monitoring of vital functions, to cardiovascular support and management of postoperative pain will provide the conditions that can contribute to the successful outcome of surgical cases.
Conflict of interest
The authors declare no conflicts of interest.
References
- Aguiar J, Mogridge G, Hall J (2014) Femoral fracture repair and sciatic and femoral nerve blocks in a guinea pig. J Small Anim Pract, 55: 635–639.
- Alworth LC, Harvey SB (2012) The laboratory rabbit, guinea pig, hamster, and other rodents. 1st ed. American College of Laboratory Animal Medicine, Academic Press (Elsevier), San Diego, pp. 955–965.
- Bannon AW, Malmberg AB (2007) Models of nociception: hotplate, tail-flick, and formalin tests in rodents. Current Protocols in Neuroscience 8, 8-9.
- Barter LS (2011) Rabbit analgesia. Vet Clin North Am Exotic Anim Practice 14, 93-104.
- Bellini L, Veladiano IA, Schrank M, Candaten M, Mollo A (2018) Prospective clinical study to evaluate an oscillometric blood pressure monitor in pet rabbits. BMC Vet Res 14, 52-60.
- Benato L, Rooney N, Murrell JC (2019) Pain and analgesia in pet rabbits within the veterinary environment: a review. Vet Anaesth Analg 46, 151-162.
- Berry HS (2015) Analgesia in the Perioperative Period. Vet Clin North Am Small Anim Pract 45, 1013-1027.
- Bourque SL, Adams MA, Nakatsu K (2010) Comparison of buprenorphine and meloxicam for post-surgical analgesia in rats: effects on body weight, locomotor activity. J Am Assoc Lab Anim Sci 49, 617–622.
- Brodbeld D (2009) Perioperative mortality in small animal anaesthesia. Vet J 182, 152-160.
- Cannon B and Nedergaard J (2011) Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 214, 242–253.
- Cao J, Du Y, Wang YJ et al. (2018) Pharmacokinetics of meperidine (pethidine) in rabbit oral fluid: correlation with plasma concentrations after controlled administration. Pharmazie, 73, 324- 328.
- Carpenter JW, Pollock CG, Koch DE (2009) Single and multipledose pharmacokinetics of meloxicam after oral administration to the rabbit (Oryctolagus cuniculus). J Zoo Wildl Med, 40, 601–606.
- Cruz F and Junquera J (1993) The immobility response elicited by clamping, bandaging and grasping in the Mongolian gerbil (Meriones unguiculatus). Behav Brain Res 54, 165-169.
- Coulter CA, Flecknell PA, Leach MC, Ritsardson CA (2011) Reported analgesic administration to rabbits undergoing experimental surgical procedures. Vet Res 7, 12-18.
- Deuis JR, Dvorakova LS, Vetter I (2017) Methods Used to Evaluate Pain Behaviors in Rodents. Front Mol Neurosci 10, 284-300.
- Dollo G, Malinovsky JM, Péron A et al. (2004) Prolongation of epidural bupivacaine effects with hyaluronic acid in rabbits. Int J Phar 19, 109-119.
- Edis A (2016) How to manage intraosseous catheters in exotic species. The Veterinary Nurse 7, 589–593.
- Egger CM, Souza MJ, Greenacre CB, Cox SK, Rohrbach BW (2009) Effect of intravenous administration of tramadol hydrochloride on the minimum alveolar concentration of isoflurane in rabbits. Am J Vet Res 70, 945-949.
- Erkert RS, MacAllister CG (2005) Use of a eutectic mixture of lidocaine 2.5% and prilocaine 2.5% as a local anesthetic in animals. J Am Vet Med Assoc 226, 1990–1992.
- Flecknell P (2018) Rodent analgesia: Assessment and therapeutics. Vet J 232, 70-77.
- Flecknell P (2002) Guinea pig. In: BSAVA manual of exotic pets. (4th edn), BSAVA, Hampshire, pp. 54-64.
- Flecknell P (2006) Anaesthesia and perioperative care. In: Meredith A, Flecknell P, eds. BSAVA Manual of Rabbit Medicine and Surgery. Quedgeley, UK, pp: 154–165.
- Fredholm DV, Carpenter JW, KuKanich B (2013) Pharmacokinetics of meloxicam in rabbits after oral administration of single and multiple doses. Am J Vet Res 74, 636–641.
- Gad SC, Spainhour CB, Shoemake C et al. (2016) Tolerable Levels of Nonclinical Vehicles and Formulations Used in Studies by Multiple Routes in Multiple Species with Notes on Methods to Improve Utility. Int J Toxicol 35, 95-178.
- Gades NM, Danneman, PJ, Wixson, SK (2000) The magnitude and duration of the analgesic effect of morphine, butorphanol, and buprenorphine in rats and mice. Contemp Top Lab Anim Sci 39, 8–13.
- Gaskil B and Garner JP (2017) Stressed out: providing laboratory animals with behavioral control to reduce the physiological effects of stress. Lab Animal 46, 142–145.
- Goodman G (2002) Hamster. In: BSAVA manual of exotic pets. (4th edn), BSAVA, Hampshire, pp. 27-33.
- Hampshire V, Robertson S (2015) Using the facial grimace scale to evaluate rabbit wellness in post-procedural monitoring. Lab Animal 4, 259-260.
- Harkness JE, Wagner JE (1995) The Biology and Medicine of Rabbits and Rodents, 4th ed, Williams & Wilkins, pp. 65-73.
- Harvey L, Knowles T, Murison P (2012) Comparison of direct and Doppler arterial blood pressure measurements in rabbits during isoflurane anaesthesia. Anesth Analg 39, 174-184.
- Hawkins MG (2006) The use of analgesics in birds, reptiles, and small exotic animals. J Exotic Pet Med 15, 177-192.
- Johnston MS (2005) Clinical Approaches to Analgesia in Ferrets and Rabbits. In: Proceedings of Seminars in Avian and Exotic Pet Medicine 14, 229-235.
- Kazakos GM, Anagnostou T, Savvas I, Raptopoulos D, Psalla D, Kazakou IM (2007) Use of the laryngeal mask airway in rabbits: placement and efficacy. Lab Anim 36, 29-34.
- Kazakos GM, Savvas I, Anagnostou T, Flouraki E, Pavlidou K, Sapanidou V (2010) Use of a eutectic mixture of lidocaine 2.5% and prilocaine 2.5% as a local anaesthetic for arterial catheterisation in rabbits. Vet Anaesth Analg 38, 27–28.
- Keating SCJ, Thomas AA, Flecknell PA, Leach MC (2012) Evaluation of EMLA Cream for Preventing Pain during Tattooing of Rabbits: Changes in Physiological, Behavioural and Facial Expression Responses. PLoS ONE 7, 1-11.
- Keeble E (2002) Gerbil. In: BSAVA manual of exotic pets. (4th edn), BSAVA, Hampshire, pp. 35-36.
- Klaus U, Weinandy R, Gattermann R (2000) Circadian activity rhythms and sensitivity to noise in the Mongolian gerbil (Meriones unguiculatus). Chronobiology International 17, 137–145.
- Kohn DF, Martin TE, Foley PL, Morris TH, M Swindle M, Vogler GA, Wixson S (2007) Guidelines for the Assessment and Management of Pain in Rodents and Rabbits, J Am Ass Lab Anim Science 46, 97-108.
- Kluge K, Larenza Menzies MP, Kloeppel H, Pearce SG, Bettschart- Wolfensberger R, Kutter AP (2017) Femoral and sciatic nerve blockades and incision site infiltration in rabbits undergoing stifle joint arthrotomy. Lab Anim 51, 54-64.
- Kula A, Akkar OB, Gulturk S, Cetin M, Cetin A (2016) Combination of paracetamol or ketamine with meperidine enhances antinociception. Hum Exp Toxicol 35, 887-892
- Langford DJ, Bailey AL, Chanda ML et al. (2010) Coding of facial expressions of pain in the laboratory mouse. Nature Methods 7, 447-449.
- Leach MC, Allweiler S, Richardson C (2009) Behavioural effects of ovariohysterectomy and oral administration of meloxicam in laboratory housed rabbit. Res Vet Sci 87, 336-347.
- Lennox AM (2008) Intraosseous Catheterization of Exotic Animals. Journal of Exotic Pet Medicine 17, 300–306.
- Lichtenberger M, Ko J, (2007) Anesthesia and analgesia for small mammals and birds. Vet Clin N Am Exotic Anim Pract 10, 293-315.
- Malinovsky, JM, Bernard JM, Baudrimont M, Dumand JB Lepage JY (1997) A chronic model for experimental investigation of epidural anesthesia in the rabbit. Regional Anesthesia, 22, 80-85.
- Meredith A (2015) BSAVA Small Animal Formulary. Part B: Exotic pets. (9th edn), BSAVA, Hampshire, UK.
- Miller AL, Leach, MC (2015) The mouse grimace scale: a clinically useful tool? PLoS ONE 10, e0136000.
- Monson CB, Oyama J (1984) Core temperature of tailless rats exposed to centrifugation. Physiologist, 27, 97-98.
- Morton D, Safron JA, Glosson J, Rice DW, Wilson DM, White RD (1997) Histologic lesions associated with intravenous infusions of large volumes of isotonic saline solution in rats for 30 days. Toxicol Pathol 25, 390–394.
- Morton DB, Jennings M, Buckwell A et al. (2001) Refining procedures for the administration of substances. Report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. British Veterinary Association Animal Welfare Foundation/Fund for the Replacement of Animals in Medical Experiments/Royal Society for the Prevention of Cruelty to Animals/Universities Federation for Animal Welfare. Lab Anim 35, 1–41.
- Navarrete-Calvo R, Gómez-Villamandos RJ, Morgaz J et al. (2014) Cardiorespiratory, anaesthetic and recovery effects of morphine combined with medetomidine and alfaxalone in rabbits.Vet Rec 174, 95-100.
- Nishiyama T (1998) A rat model of chronic lumbar epidural catheterization. Can J Anaesth, 45, 907–912.
- Orr HE (2002) Rats and Mice. In: BSAVA manual of exotic pets. (4th edn), BSAVA, Hampshire, pp. 16-17.
- Owens NC, Ootsuka Y, Kanosue K, McAllen RM (2002) Thermoregulatory control of sympathetic fibres supplying the rat’s tail. J Physiol 543, 849–858.
- Quesenberry K and Carpenter J (2012) Ferrets, Rabbits, and Rodents, Clinical Medicine and Surgery (3rd ed). Elsevier, Missouri, pp. 429-451.
- Roughan JV and Flecknell PA (2003) Evaluation of a short duration behaviour-based post-operative pain scoring system in rats. Eur J of Pain 7, 397-406.
- Smiler KL, Stein S, Hrapkiewicz KL, Hiben JR (1990) Tissue response to intramuscular and intraperitoneal injections of ketamine and xylazine in rats. Lab Anim Sci 40, 60–64.
- Souza MJ, Greenacre CB, Cox SK (2008) Pharmacokinetics of orally administered tramadol in domestic rabbits (Oryctolagus cuniculus), Am J Vet Res 69, 979-982.
- Stokes EL, Flecknell PA, Richardson CA (2009) Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab Anim 43, 149-154.
- Swisher S, Lennox A (2015) Analgesia in Small Exotic Mammals: A review. Advances in Small Animal Medicine and Surgery 28, 1-3.
- Tran AN and Koo JY (2014) Risk of systemic toxicity with topical lidocaine/prilocaine: a review. J Drugs Dermatol 13,118-122.
- Tranquilli WJ, Thurmon JC, Grimm, KA (2007) Lumb and Jones’ Veterinary Anesthesia and Analgesia, 4th ed, Blackwell, Iowa, USA, pp. 765-785.
- Turner PV, Brabb T, Pekow C, Vasbinder M (2011) Administration of Substances to Laboratory Animals: Routes of Administration and Factors to Consider. J Am Assoc Lab Anim Sci 50, 600–613.
- Uchida S, Hara K, Kobayashi A, Funato H, Hobara T et al. (2010) Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J Neurosci Res 30, 15007–15018.
- Van den Hoogen RH, Colpaert FC (1981) Long term catheterization of the lumbar epidural space in rats. Pharmacol Biochem Behav 15, 515–516.
- Wagner JE (2014) The Biology of the Guinea Pig. Academic Press, pp. 5- 13.
- Wenger S (2012) Anesthesia and analgesia in rabbits and rodents. Journal of Exotic Pet Medicine 21, 7–16.
- Wilson DM, Romero JC, Strong CG et al. (1975) Indirect blood pressure measurements in the rabbit: correlations with direct aortic and ear pressures. J Lab Clin Med 86, 1032–1039.
- Woolf, CJ and Chong MS (1993) Preemptive analgesia–treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 77, 362–379.
- Wurbel H (2001) Ideal homes? Housing effects on rodent brain and behavior. Trends in Neuroscience 24, 207- 211.
- Ypsilantis, P, Didilis, VN, Politou, M et al. (2000) A comparative study of invasive and oscillometric methods of arterial blood pressure measurement in the anesthetized rabbit. Res Vet Sci 78, 269–275.
Corresponding author:
Panagiota Karamichali
e-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.



