> Abstract
Anal furunculosis is a chronic progressive canine inflammatory disorder affecting the anus and the perianal region, resulting in ulcers and blind fistula in the skin and subcutaneous tissues of the perianal region. German shepherds are predisposed to this condition. The origins of the disorder are unknown but it can be immune- mediated. Treatment can be medical or surgical, but complete cure is uncommon. Medical treatment with immunomodulatory drugs, mostly cyclosporine or cyclosporine and ketoconazole usually provides satisfactory results. Surgical treatment is employed in cases with no response to medical management, due to increased cost or prolonged duration of the latter and includes complete anoplasty and simultaneous anal sacculectomy.
 > Introduction
> Introduction
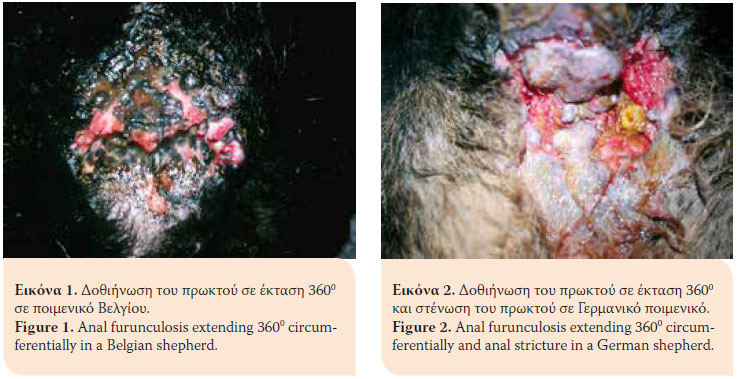
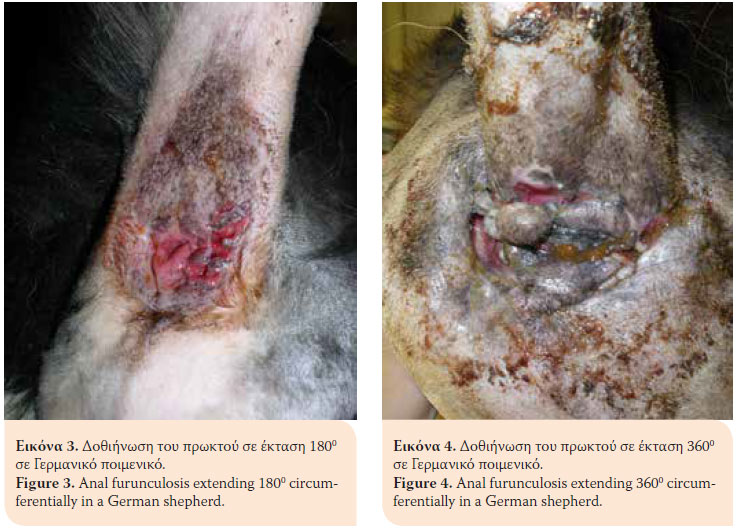
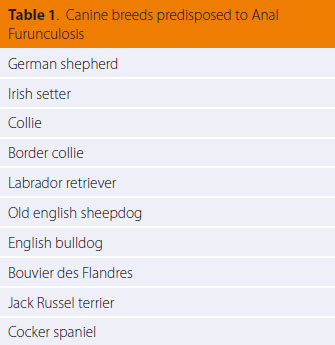
Anal furunculosis (AF) or perianal fistulae is a chronic progressive canine inflammatory disorder of dogs affecting the anal orifice and the perianal region. It is characterised by the presence of ulcerative lesions and blind fistulae in the skin and subcutaneous tissues of the perianal and perineal regions.1 The sinus tracts can be single or multiple and they can extend 3600 circumferentially in the perianal area (Figures 1,2,3,4).2 AF affects dogs with a median age of six years and of both genders. German shepherds are predisposed to the disorder even though AF occurs in other middle or large sized breeds as well as mixed breed dogs (Table 1).1,3-5
> Pathogenesis
The precise origin of the disorder is not fully known. Recent studies implicated an inflammatory response with presence of T-lymphocytes, other inflammatory cells, cytokines, enzymes, and other intermediaries of the inflammatory cascade and histological similarities between AF and Crohn’s disease in people, resulting in the theory of a possible immune-mediated origin.6-11 This theory is supported by an impressive response following administration of immune-mediated drugs.2 German shepherd breed predisposition to AF is probably of genetic origin, contributing to the pathogenesis of AF.12
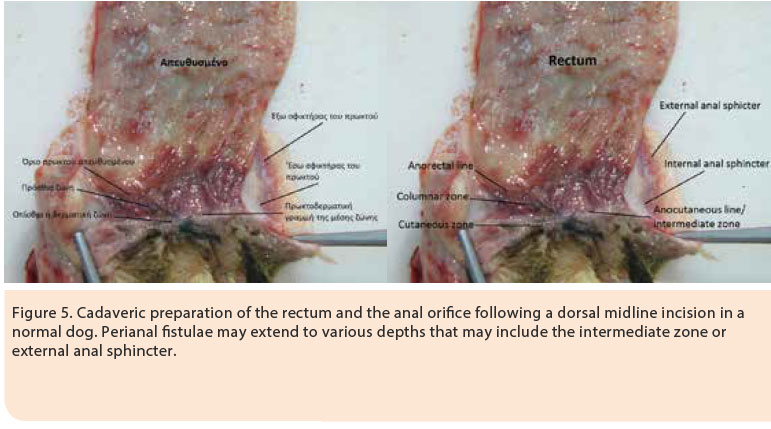
Originally, AF manifests as a subtle inflammatory response without the presence of ulcers. At this time, ulcerative lesions and sinus tracts are formed, which are lined by squamous epithelium and they are infiltrated by lymphocytes, plasmacytes, macrophages, neutrophils and eosinophils. The evolution of the inflammatory response is characterised by the presence of Τ-lymphocytes with extensive formation of granulomatous and fibrous tissue (fibrosis). The normal anatomy of the area is disrupted with the formation of fistulae originating from the anal glands to the anocutaneous junction, as well as lesions in the anal sacs (Figure 5). The contribution of the anal sacs to the inflammatory response is secondary resulting in abscessation and rupture. The formation of fibrous tissue in the area of the outer anal sphincter in dogs with extensive and severe lesions can result in stricture. 3,13
 >Clinical signs
>Clinical signs
Clinical signs in dogs with AF vary and are usually connected with pain in the perineal area and disorders of defecation (Table 2).1,14-16 Several dogs already have severe lesions at the time of diagnosis.14
> Diagnosis
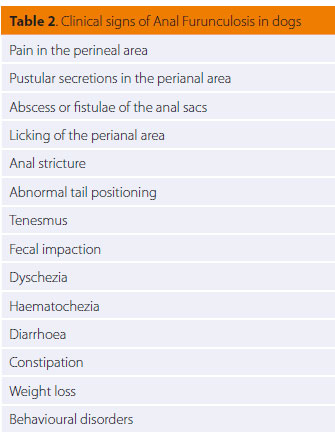
Reaching a diagnosis of AF is mostly based on the history and physical examination findings. It is necessary to perform a physical examination, both with the dog in an alert state and under general anaesthesia. In the alert state, other than external examination of the anus and the perianal area, the external anal spincter tone is also tested. Most of the times, however, physical examination in the alert state is difficult or even impossible due to severe pain elicited both by palpation of the area as well as from handling and elevating the tail. Prior to administrating general anaesthesia, it is necessary to obtain a complete blood count, serum biochemistry analysis and urinalysis, as part of the preanaesthetic evaluation, as well as to exclude other conditions with similar clinical signs. Then the perianal region is visually observed to assess whether the diseased areas may in part (0ο- 270ο) or entirely (360ο) affect the region. Rectal digital palpation is necessary in order to locate and evaluate the formation of fibrous tissue and the degree of rectal fibrosis. Stricture formation may lead to constipation and obstipation (Figure 6). Palpation of the anal sacs is also performed by digital rectal palpation and patency of their excretory ducts is determined; any sinus tracts connecting the perianal region with the anal glands are detected and the contribution of the anal glands to the inflammatory process is assessed (Figure 7). The depth of the sinus tracts is determined by using a probe. Colonoscopy and biopsy should be performed in some dogs with clinical signs of colitis, as a strong correlation has been found between chronic inflammatory disease of the large intestine and AF.17-20
> Differential diagnosis
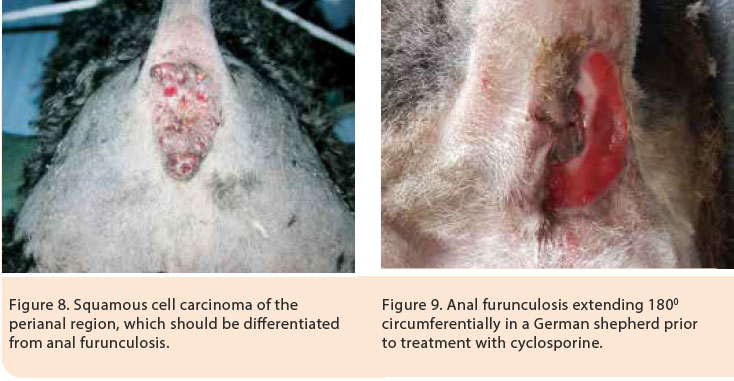
AF should be differentiated from abscessation and rupture of the anal glands, anal gland adenocarcinoma, adenoma and adenocarcinoma of the perianal glands, squamous cell carcinoma of the perianal region, chemical burns, trauma and subcutaneous mycosis (Figure 8).2 In such cases and when in doubt, cytological and histopathological examinations are recommended.
> Treatment
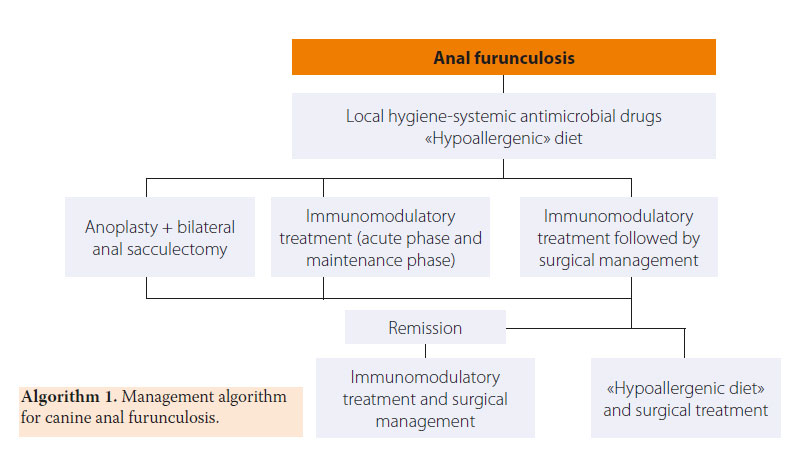
Treatment for AF can be medical or surgical, but it is uncommon to lead to complete and permanent cure. Surgical management of AF has been the treatment of choice for several years. A multitude of surgical techniques have been used in the past, such as surgical excision of fistulae, surgical debridement combined with chemical cauterisation, cryosurgery, cauterisation by electrodiathermy, complete tail amputation (given that a wide tail was implicated as a predisposing factor for AF) and excision by ND:YAG laser. The high percentage of relapsing and complications, including faecal incontinence, surgical wound dehiscence and anal stricture, have led to abandonment of surgical treatment as a single management strategy for AF. Moreover, many dogs required several surgical procedures in order to correct any complications.1,4,14,15,21-26
> Medical management
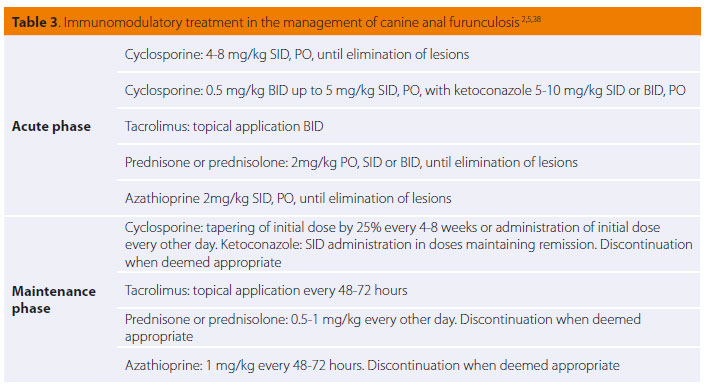
Nowadays medical management with immunomodulatory drugs is considered to be the treatment of choice due to highly satisfactory results.2 Medical treatment aims in suppressing the pain and lesions of the anal orifice and perianal area both in the short and long-term, as relapses of the disease after treatment is withdrawn are not uncommon. Medical management includes the acute phase, the maintenance phase, a specialised clinical diet and local perineal hygiene-antimicrobial treatment. For the acute and maintenance phase cyclosporine has been used as monotherapy or in combination with ketoconazole, glucocorticoids, azathioprine and local application of tacrolimus (Table 3).2,27-39 Τhis medication can lead to remission and, in some cases, elimination of lesions and clinical cure.27-37
Glucocorticoids mostly suppress cellular immunity, are inexpensive but their administration usually results in adverse effects (polyuria, polydipsia, and polyphagia). Initially they had been used combined with a specialised diet in the management of AF. In 27 German shepherds prednisone was administered (2 mg/kg/SID [/24 hours] PO [orally]) for 2 weeks, then 1mg/kg for 4 weeks and then was reduced to at a dose of 1mg/kg/48h for 8-16 weeks. Clinical cure was achieved in 33.3% of dogs, improvement in 33.3% and the rest did not respond to medical treatment.27
Azathioprine has been used successfully in the management of AF. Due to the long period of time that is necessary for this drug to be fully effective, it is recommended to combine it with glucocorticoids, at least during the acute phase.38 In a prospective study with 14 dogs with AF a combination of azathioprine and prednisone was administered. Permanent cure was achieved in 57% of cases, partial improvement in 7% and no response in 36% of dogs.40
Cyclosporine is a powerful immunomodulatory agent, which suppresses the production of inflammatory cytokines, which are related to the activation of T-lymphocytes. In particular, cyclosporine suppresses mostly the production of interleukin-2, which is necessary for the differentiation and proliferation of T-lymphocytes.39 The administration of cyclosporine is considered the most effective treatment of AF with a success rate of 50-85%.29,30,33,35,36
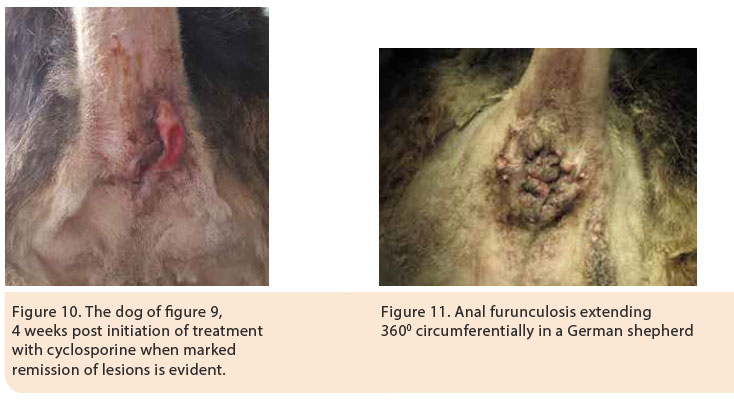
The mean duration of treatment until the eradication of lesions, according to one study, was 8.8 weeks.35 However, discontinuation of treatment may result in relapse usually necessitating continuation of life-long treatment at the smallest dose possible. 28 Cyclosporine was initially administered at a dose regimen of 4-8 mg/kg SID PO for 2-4 months, until the eradication of lesions, and then the dose was gradually tapered by 25% every 4-8 weeks or alternatively the initial challenge dose was administered every other day after remission of clinical signs (Figures 9, 10).2,35,38 Cyclosporine treatment is costly and can be associated with adverse effects including vomiting, diarrhoea, anorexia, lethargy, aggression, hypertrichosis or trichorrhoea.29,33,35,36
Τhe high cost of cyclosporine directed many researchers to investigate alternate methods of treatment. The combination of cyclosporine with ketoconazole reduces the cost of treatment with no change in effectiveness, compared to monotherapy with cyclosporine.41 Ketoconazole, an antifungal agent, affects the metabolism of cyclosporine by inhibiting the effect of cytochrome P450 3A oxidase, resulting in the increase of cyclosporine blood serum levels.41-43 Cyclosporine was administered at a dose regimen ranging from 0.5 mg/kg BID (/12 hours) up to 5 mg/kg PO SID and ketoconazole in a dose of 5-10 mg/kg PO SID.2,38,41-43 According to a study in 19 dogs with AF, the combination of cyclosporine and ketokonazole was successful in eradicating lesions when used for a duration of 3-10 weeks in 100% of dogs, whereas relapse was observed in 37% of dogs after a period of 1-6 months after the initial treatment.41
Tacrolimus is a topically applied macrolide with a similar immunomodulatory effect to that of cyclosporine, which has been used successfully in the management of AF.2,31,37 In a study of 10 dogs with AF, the topical application of tacrolimus SID or BID for 16 weeks resulted in remission of lesions in 90% of dogs, among which 50% were completely cured.31 In a different recent study of a total duration of two years, in 19 dogs with AF, 0.1% tacrolimus ointment was simultaneously administered with prednisone (2 mg/kg SID over two weeks, 1mg/kg SID for four weeks and 1mg/kg/48h for ten weeks) in combination with “hypoallergenic” diet. Moreover, metronidazole was administered PO at a dose of 10mg/kg BID for two weeks. After the completion of 16 weeks, 79% of dogs were cured and in 21% significant improvement was observed. During the next two years, the maintenance regimen was adhered to with tacrolimus and prednisone, and 86.6% of dogs apparently remained in remission.37 Tacrolimus is recommended mostly for long-term maintenance, following initial remission of clinical signs with administration of cyclosporine, when applicable, every 24-72 hours aiming at the prevention of relapses.2,38 Τhis treatment is considered to be costly.2,37,38
> Clinical Diet
The combination of AF and colitis prompted several authors to recommend a specialised “hypoallergenic” diet with hydrolysed protein or protein to which the dog has never been exposed previously. Thirty three dogs with AF were fed a diet based on fish and potatoes, for 1-180 days prior to surgery (en bloc resection of lesions and anoplasty) and one year later 87.9% of dogs experienced a complete eradication of lesions, whereas only 20.7% manifested any clinical signs.16 A specialised “hypoallergenic” diet is therefore recommended during the maintenance phase, especially in cases of lesion recurrence.2,38
> Local hygiene and systemic antimicrobial treatment
Local cleansing of the perineal region and hygiene measures such as clipping of the hair coat and lavage with antiseptic solutions along with systemic and topical administration of antibiotics, following culture and sensitivity testing, could aid in reducing the local bacterial flora and managing the secondary bacterial infections that are always present.2,38
> Preoperative immunomodulatory treatment
Preoperative immunomodulatory treatment results in remission of lesions allowing for a more conservative approach during surgical resection and reduction of complications to a minimum.30 Various treatment regimens have been employed, such as the administration of azathioprine (50 mg PO) and metronidazole (400 mg PO) for a duration of 4-6 weeks preoperatively and two weeks postoperatively. A significant clinical improvement was noted during the first two weeks, but after the advent of 4-6 weeks there was minimal further improvement. Dogs that received the above regimen did not relapse in the following 7-10 months.19 The adverse effects of azathioprine include gastrointestinal disorders, bone marrow suppression, hepatotoxicity, and pancreatitis whereas metronidazole side effects include anorexia, central nervous system toxicity and hepatotoxicity.2 In a study of 25 dogs with AF, cyclosporine was administered (2.5-5 mg/kg BID PO) as monotherapy, cyclosporine (1-1.5 mg/kg BID PO) in combination with ketoconazole (12.5 mg/kg SID PO), or azathioprine (1-2 mg/kg SID PO) in combination with prednisolone (1 mg/kg BID PO for 2 weeks and then reduced to 0.5 mg/kg BID PO) for less than 12 weeks. Surgical management included resection of all fistulae, cryptectomy of the transitional zone of the rectum up to the anocutaneous line and bilateral anal sacculectomy. None of the dogs relapsed after a follow-up time of 9 months after surgery.30
> Surgical management
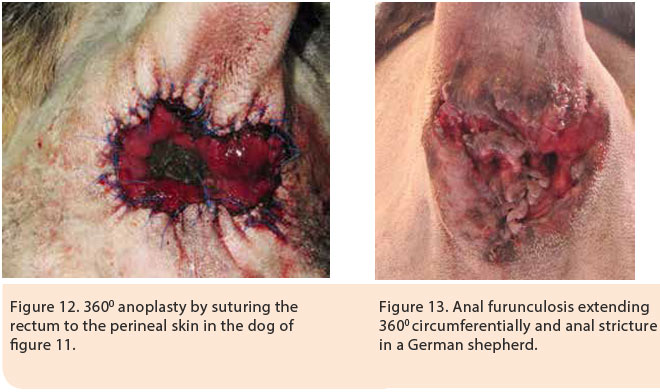
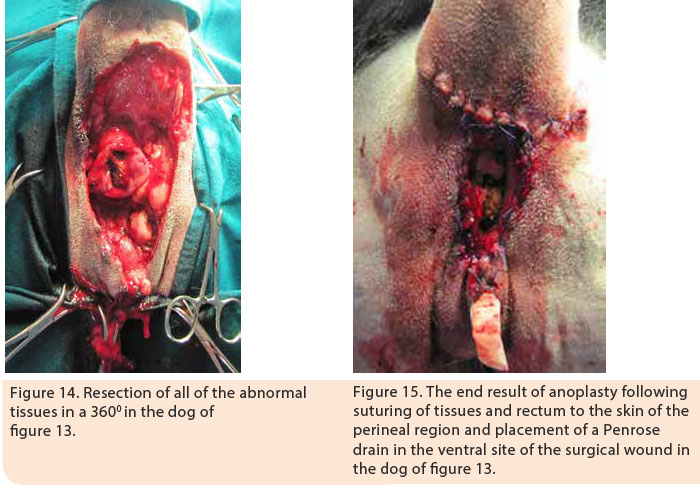
Indications of surgical management include cases nonresponsive to medical treatment, cases with AF affecting the anal glands, and cases in which the cost of medical treatment is significant and its duration is prolonged. Surgical resection of all of the affected tissues is a prerequisite for successful results. 1,5,16 Good knowledge of anal canal anatomy is necessary in order to prevent severe complications. Surgical resection includes the removal of all perianal fistulae and their sinus tracts, as well as bilateral anal sacculectomy due to anal sac implication in the pathogenesis of the disorder.5,16 Currently, in cases of extensive lesions in the perianal region, complete 3600 anoplasty is recommended with simultaneous bilateral sacculectomy.5,16 Following surgical resection of the skin around the anus, all of the lesions are removed including the subcutaneous tissue, the muscles and fascia as well as the anal orifice. If necessary, partial or complete resection of the external anal sphincter is performed. Dead space is eliminated and the subcutaneous tissue is approximated to the serosa and muscular layer of the rectum by 3/0 polydioxanone simple interrupted sutures. Finally, the mucosal layer and submucosa of the rectum are sutured to the skin by 3/0 nylon sutures (Figures 11,12, 13, 14, 15).5,16 In cases when surgical wound closure is impossible, due to increased tension, healing by secondary intention is preferred. In a study of 51 dogs with AF which underwent 3600 anoplasty combined with bilateral anal sacculectomy, after a median follow-up of 18 months, 2% of cases relapsed, 13% manifested anal stenosis and 4% had fecal incontinence.5
Treatment of AF is summarised in Figure 1.
> References
1. Harvey CE. Perianal fistula in the dog. Vet Rec 1972, 91: 25-33
2. Patterson AP, Campbell KL. Managing anal furunculosis in dogs. Compend Contin Educ Pract Vet 2005, 27: 339-355
3. Day MJ, Weaver BMQ. Pathology of surgically resected tissue from 305 cases of anal furunculosis in the dog. J Small Anim Pract 1992, 33: 583-589
4. Vasseur PB. Results of surgical excision of perianal fistulas in dogs. J Am Vet Med Assoc 1984, 185: 60-62
5. Milner HR. The role of surgery in the management of canine anal furunculosis. A review of the literature and a retrospective evaluation of treatment by surgical resection in 51 dogs. New Zealand Vet J 2006, 54: 1-9
6. House A, Gregory SP, Catchpole B. Expression of cytokine mRNA in canine anal furunculosis lesions. Vet Rec 2003, 153: 354-358
7. House AK, Catchpole B, Gregory SP. Matrix metalloproteinase mRNA expression in canine anal furunculosis lesions. Vet Immunol Immunopathol 2007, 115: 68-75
8. House AK, Gregory SP, Catchpole B. Pattern- recognition receptor mRNA expression and function in canine monocyte/macrophages and relevance to canine anal furunculosis. Vet Immunol Immunopathol 2008, 124: 230-240
9. Tivers MS, Catchpole B, Gregory SP, House AK. Interleukin-2 and interferon-gamma mRNA expression in canine anal furunculosis lesions and the effect of ciclosporin therapy. Vet Immunol Immunopathol 2008, 125: 31-36
10. 1House AK, Binns MM, Gregory SP, Catchpole B. Analysis of NOD1, NOD2, TLR1, TLR2, TLR4, TLR5, TLR6 and TLR9 in anal furunculosis of german shepherd dogs. Tissue Antigens 2008, 73: 250-254
11. Barnes A, O’Neil T, Kennedy LJ, Short AD, Catchpole B, House A, Binns M, Fretwell N, Day MJ, Ollier WER. Association of canine anal furunculosis with TNFA is secondary to linkage disequilibrium with DLA-DRB1. Tissue Antigens 2008, 73: 218-224
12. Kennedy LJ, O’Neil T, House A. Barnes A, Kyöstilä K, Innes J, Fretwell N, Day MJ, Catchpole B, Lohi H, Ollier WE. Risk of anal furunculosis in German shepherd dog is associated with the major histocompatibility complex. Tissue Antigens 2008, 71: 51-56
13. Killingsworth CR, Walshaw R, Dunstan RW, Rosser EJ. Bacterial population and histologic changes in dogs with perianal fistula. Am J Vet Res 1988, 49:1736-1741
14. Robins GM, Lane JG. The management of anal furunculosis. J Small Anim Pract 1973, 14: 333-342
15. Houlton JEF. Anal furunculosis: a review of seventy cases. J Small Anim Pract 1980, 21: 575-584
16. Lombardi RL, Marino DJ. Long- Term Evaluation of canine perianal fistula disease treated with exclusive fish and potato diet and surgical excision. J Am Anim Hosp Assoc 2008, 44: 302-307
17. Jamieson PM, Simpson JW, Kirby BM, Else RW. Association between anal furunculosis and colitis in the dog: preliminary observations. J Small Anim Pract 2002, 43:109-114.
18. Massey J, Short AD, Catchpole B, House A, Day MJ, Lohi H, Olier WE, Kennedy LJ. Genetics of canine anal furunculosis in the German shepherd dog. Immunogenetics 2014, 66: 311-324.
19. Tisdall PLC, Hunt GB, Beck JA, Malik R. Management of perianal fistulae in five dogs using azathioprine and metronidazole prior to surgery. Aust Vet J 1999, 77: 374-378
20. Lombardi RL, Marino DJ. Long- term evaluation of canine perianal fistula disease treated with exclusive fish and potato diet and surgical excision. J Am Anim Hosp Assoc 2008, 44: 302-307
21. Lane JG, Burch DGS. The cryosurgical treatment of canine anal furunculosis. J Small Anim Pract 1975; 16: 387-392
22. Houlton JEF. Canine anal furunculosis: a modified approach. J Small Anim Pract 1980, 21: 585-593
23. Elkins AD, Horbson HP. Management of perianal fistulae a retrospective study of 23 cases. Vet Surg 1982, 11: 110-114
24. Goring RL, Bright RM, Stancil ML. Perianal fistulas in the dog. Retrospective evaluation of surgical treatment by deroofing and fulguration. Vet Surg 1986, 15: 392-398
25. Van Ee RT, Palmitieri A, Tail amputation for treatment of perianal fistulas in dogs. J Am Anim Hosp Assoc 1987; 23: 95-100
26. Ellison GW, Bellah JR, Stubbs WP, Gilder JV. Treatment of perianal fistulas with ND: YAG laser- results in twenty cases. Vet Surg 1995, 24: 140-147
27. Harkin KR, Walshaw R, Mullaney TP. Association of perianal fistula and colitis in the German shepherd dog: response to high-dose prednisone and dietary therapy. J Am Anim Hosp Assoc 1996, 32: 515-520.
28. Mathews KA, Ayres SA, Tano C, Riley SM, Sukhiani HR, Adams C. Cyclosporin treatment of perianal fistulas in dogs. Can Vet J 1997, 38: 39-41
29. Mathews KA, Sukhiani HR. Randomized controlled trial of cyclosporine for treatment of perianal fistulas in dogs. J Am Vet Med Assoc 1997, 211: 1249-1253
30. Klein A, Deneuche A, Fayolle P, Hidalgo A, Scotti S, Zylberstain L, Desbois C, Tessier D, Moissonnier P, Viateau V. Preoperative immunosuppressive therapy and surgery as a treatment for anal furunculosis. Vet Surg 2006, 35: 759-768
31. Misseghers BS, Binnington AG, Mathews KA. Clinical observations of the treatment of canine perianal fistulas with topical tacrolimus in 10 dogs. Can Vet J 2000, 41: 623-627
32. Griffiths LG, Sullivan M, Borland WW. Cyclosporine as the sole treatment for anal furunculosis: preliminary results. J Small Anim Pract 1999, 40: 569-572
33. Doust R, Griffiths LG, Sullivan M. Evaluation of once daily treatment with cyclosporine for anal furunculosis in dogs. Vet Rec 2003, 152: 225-229
34. O’Neill T, Edwards GA, Holloway S. Efficacy of combined cyclosporine A and ketoconazole treatment of anal furunculosis. J Small Anim Pract 2004, 45: 238-243
35. Hardie RJ, Gregory SP, Tomlin J, Sturgeon C, Lipsomb V, Ladlow J. Cyclosporine treatment of anal furunculosis in 26 dogs. J Small Anim Pract 2005, 46: 3-9
36. House AK, Guitian J, Gregory SP, Hardie RJ. Evaluation of the effect of two dose rates of cyclosporine on the severity of perianal fistulae lesions and associated clinical signs in dogs. Vet Surg 2006, 35: 543-549
37. Stanley BJ, Hauptman JG. Long-term prospective evaluation of topically applied 0,1% tacrolimus ointment for treatment of perianal sinuses in dogs. J Am Vet Med Assoc 2009, 235: 397-404
38. Pieper J, McKay L. Perianal fistulas. Compend Contin Educ Vet 2011, 33: E1-E4
39. Guaguere E, Steffan J, Olivry T. Cyclosporin A: a new drug in the field of canine dermatology. Vet Dermatol 2004, 15: 61–74
40. Harkin KR, Phillips D, Wilkenson M. Evaluation of azathioprine on lesion severity and lymphocyte blastogenesis in dogs with perianal fistulas. J Am Anim Hosp Assoc 2007, 43: 21-26
41. O’Neill T, Edwards GA, Holloway S. Efficacy of combined cyclosporine A and ketoconazole treatment of anal furunculosis. J Small Anim Pract 2004, 45: 238-243
42. Patricelli AJ, Hardie RJ, McAnulty JE. Cyclosporine and ketoconazole for the treatment of perianal fistulas in dogs. J Am Vet Med Assoc 2002, 220: 1009-1016
43. Mouatt JG. Cyclosporin and ketoconazole interaction for treatment of perianal fistulas in the dog. Aust Vet J 2002, 80: 207-211



