> Abstract
Pleural effusions constitute a common entity in feline medicine. Laboratory evaluation of pleural fluids remains the cornerstone of a proper diagnosis. Total nucleated cell count, total protein concentration and haematocrit values are the most important indices, which, along with cytological findings, are used for the classification of an effusion (transudate, modified transudate, exudate, haemorrhagic effusion). Occasionally, the biochemical examination of effusions is necessary in order to determine the presence of fluid of a more specific aetiology (chyle, feline infectious peritonitis effusions, septic exudates), while microbiological examination remains a standard procedure in suspected septic effusions. After having considered the obtained information, clinicians are usually able to understand the aetiology behind cavitary fluid accumulation and thus make the best therapeutic decision for the cat patient.
> Introduction
Amongst the most common pathologic conditions of the pleural cavities, as those are defined by the serous membranes that cover the lungs and the inner surface of the thoracic walls, are the accumulation of either abnormal quantities of fluid (termed as pleural effusion) or air (termed as pneumothorax). In feline medicine, pleural eff usions are the second most frequent cause of respiratory distress, exceeded only by heart disease.1,2,3 The diagnosis of an eff usion is considered challenging in cat patients as cats tend to compensate and quickly resume their normal activity. The presence of a pleural fluid is confirmed by diagnostic imaging while in cases of acute respiratory distress, stabilizing measures such as oxygen supply and alleviating thoracocentesis are required. Fluid aspiration is performed with the cat placed in sternal recumbency by inserting either a butterfl y needle (20-22 G) or an over-the-needle catheter (20-22 G) connected with a 3-way tap and a 10-50 ml syringe, into the 7th or 8th intercostal space.4 Laboratory investigation of feline pleural eff usions is essential and is not greatly diff erentiated from that of dog eff usions, with the exception of feline infectious peritonitis (FIP). The laboratory evaluation of feline pleural fluids off ers valuable information on the pathogenesis of eff usion formation which subsequently facilitates final diagnosis or narrows down the diff erential diagnosis. However, the in-clinic evaluation of eff usions requires certain basic laboratory equipment, such as an automated haematology analyser or haemocytometer, a centrifuge and a light microscope.
The present study is a review of routine laboratory investigation conducted in cats with pleural effusions.
> Sample handling
The fluid aspirated via thoracocentesis is separated into different aliquots which are subsequently placed in K3-Ethylene Diamine Tetraacetic Acid (K3-EDTA) tubes, serum tubes and/or sterile plain tubes.52 Samples should be placed in EDTA tubes shortly after aspiration in order to prevent their clotting and should then be submitted for total nucleated cell count (TNCC), total protein (TP) and specific gravity (SG) evaluation, as well as for smear preparation. Failure to prevent sample clotting might lead to false TNCC and cytological smears of poor quality.5 Other coagulants, such as Lithium-Heparin tubes should not be used as alternatives since certain cytological features of the sample might be altered.6 At this point, it must be noted that direct smears should always be prepared within 30 minutes of fluid collection in order to avoid sample deterioration and potential cytological misinterpretation.7
Aliquots placed in serum tubes are submitted for biochemical examination of the fluid. Samples should be centrifuged at 3,000 rpm for 5 minutes and the supernatant is then submitted for evaluation. It should be noted that when glucose (GLU) concentration needs to be determined (see Biochemical analysis), the supernatant should be separated within 30-60 minutes of sample collection.8 Hazy supernatants may interfere with the results obtained for several analytes. Sterile plain tubes are used for microbiological examination (bacterial/fungal cultures, susceptibility tests) in cases suspected of septic inflammation or microbial contamination. In the event of suspected anaerobic infection, the sample should be collected with minimum oxygen contamination and subsequently placed in an anaerobic bacteria transport medium.9 Samples placed in EDTA tubes are inappropriate for bacteriological culture due to the bacteriostatic and bactericidal properties of EDTA.8 Cytological examination of the fluid requires a minimum of two smears per sample, direct or sediment; the latter pertains to fluids of low cellularity (recommended in fluids with less than 3x109 cells/L). Instead of sediment smears (by means of sample centrifugation or a crafted sample chamber), cytospin smears could be prepared. Buffy-coat smear preparation might be proven useful in the evaluation of haemorrhagic or neoplastic effusions.8 On the other hand, fluids of high cellularity are strictly examined as direct smears.8
Technically, smears from fluids are either prepared as line or feathered-edge smears. In line smears, the slide is lifted up abruptly covering a short distance allowing a line of fluid to be created that is expected to have a profusion of cells.7,8 After being air-dried and fixed with common fixatives (e.g. methanol), the smears are either stained with a Romanowsky- type stain (e.g. Giemsa) if they are to be evaluated in-clinic, or are preferably saved unstained if they are to be submitted to an external laboratory.
> Clinicopathological investigation of feline pleural effusions
Taking into account the physical and biochemical properties of the sample, as well as the absolute and the differential cell count along with the TP and SG values, pleural eff usions are classified into pure or modified transudates, septic or non-septic exudates, of lymph, blood and neoplastic origin (Table 1).8 Biliothorax has been previously reported in a Siamese cat as a post-thoracostomy complication.10 While this classification discloses the pathogenesis of the fluid accumulation, little information is gained in terms of aetiological diagnosis.11
Macroscopic evaluation
Initially, fluid samples are evaluated macroscopically as to their colour, turbidity, odour and clot formation.5 As a general rule, colourless and clear samples are suggestive of fluids of low cellularity (transudates), yellowish to pink samples with a clear to slightly turbid appearance are usually indicative of fluids of low to moderate cellularity (modifi ed transudate), whereas a turbid to opaque appearance suggests fluids of moderate to high cellularity (exudates).8 A green-brown sample, generally accompanied by a foul smell, is typical of a septic exudate, although odourless samples are not always bacteria-free.5,12,13 Tints of red colour suggest blood contamination of the sample, while serosanguinous or pure red colour is compatible with haemothorax.5,11,14
A lactescent fluid is highly suggestive of chylothorax.5 In some cases of confirmed chylothorax, colourless to serosanguinous fluid has also been obtained.11 The diff erentiation of chylous from pseu- dochylous effusions rests mainly on a biochemical and cytological basis (see Biochemical analysis and Cytological evaluation), although pseudochylous effusions are usually not milky white but opaque, because of cell debris content.5 However, it should be noted that chylous effusions from anorectic animals might be opaque rather than milky.7 To define the presence of fi brinogen in effusions, a rather simple test can be performed by placing a small amount of sample into a serum tube and observing any clot formation.15 Threads or flakes of fibrin may be reported in exudates, chylous or blood effusions, indicating elevated TP concentration.5,14 Notably, bloody samples rarely clot.12,14
Gross evaluation of supernatant after centrifugation is also important, especially in turbid, bloody or lactescent samples.12 In cases of turbid or milky samples, if turbidity dissolves after centrifugation, cells or debris are considered to have imparted this turbid appearance to the sample (pseudochylothorax). On the other hand, if turbidity persists after centrifugation, a triglyceride-rich effusion is most likely (chylothorax).12,16 Additionally, in chylothorax cases after centrifugation, a characteristic “creamy” layer of chylomicrons may develop on top of the fluid.5 In bloody samples, the supernatant may present either a either typical plasma appearance, clear or with a grade of a haemolysis if acute or chronic haemorrhage is the primary cause, or a yellowish hue in chronic effusions or if red blood cell breakdown has occurred.17
Feline infectious peritonitis eff usions are characterized by specifi c macroscopic features common to eff usions with elevated TP values: frothing when agitated, clot formation even in EDTA tubes, high viscosity and clear yellow colour.11,13,17,18 Nonetheless, other pathologic conditions should be excluded before making an FIP diagnosis, even though the fluid seems typical.18
Cell counts
Cell counts in eff usions are typically performed on automated haematology analysers. Some in-clinic haematology analyzers can yield TNCC with considerable accurancy.19,20 Nevertheless, automated cell counts cannot replace the cytological examination of the fluid as results do not always correlate well with cytological appearance.5 Alternatively, a haemocytometer might be used.5,16
The absolute cell count comprises TNCC, red blood cell count (RBCC) and platelet count (PLTC).17 The TNCC, along with TP/SG values and the respective cytological features allow a primary classifi cation of eff usions into transudates, modifi ed transudates and exudates.7 In general terms, transudates and modifi ed transudates are characterized by low TNCC (TNCC <1.5 x 109cells/L), whereas exudates consistently exhibit high TNCC (TNCC >5 x 109cells/L).5 Feline infectious peritonitis eff usions are mostly considered as exudates, although they have low TNCC.5 When performed on a haematology analyser, TNCC not only includes WBC but also cells like mesothelial cells, macrophages and neoplastic cells.1,12 Thus, microscopic examination of the sample is necessary in order to identify the cells present. Red blood cell counts, haematocrit (Hct) values and PLTC depict the presence of blood in the sample. In the event that an automated method is unavailable, packed cell volume (PCV) might be performed by means of Wintrobe or microhaematocrit tubes.21 If the presence of blood is due to acute haemorrhage, the Hct or PCV of fluid is similar to the respective of the peripheral blood.7 However, in chronic haemorrhages, Hct or PCV values usually decline.21 Effusions with peripheral blood contamination due to accidental laceration of capillaries during fluid aspiration are characterized by low Hct or PCV values.5
Automated or manual differential cell count yields information concerning the presence and type of inflammation. The respective cell categories that are included in the count are all nucleated cells (mononuclear cells, polymorphonuclear cells) and they are usually the same as in CBCs. Consequently, an acute inflammatory process is characterized by high neutrophil counts, while chronic effusions mostly yield high large mononuclear (macrophages) and small lymphocyte counts. Blood contamination of the sample should be considered before a differential cell count is performed.
Total Proteins (TP) and Specific Gravity (SG)
Determination of TP values in feline effusions by means of refractometry is considered a reliable method, although dry chemistry analyzers generally afford higher accuracy for TP determination.5,22 Urine strips have also been used for in-house determination of TP concentrations below or above 20 g/L.23 Total protein and SG constitute indices of ongoing inflammation and its intensity.11 Elevated TP values (>25 g/L) usually suggest modified transudates or exudates, whereas low TP values (<25 g/L) are indicative of transudates. Should an effusion not clearly fall into one of the three main categories, TP concentration is considered more reliable for the differentiation of transudates from modified transudates, while TNCC is considered more reliable for the differentiation of modified transudates from exudates.15 Feline infectious peritonitis effusions are mostly considered as exudates.5
Specific gravity values are determined by means of refractometry and roughly depict the solute concentration of the fluid. However, SG values typically apply to urine analysis and are not diagnostically sensitive in the case of pleural effusions.21 Determination of TP and SG values should be performed in the sample’s supernatant, as the fluid’s turbidity might lead to falsely elevated TP values by refractometry or spectrophotometry.5,11 Lactescent samples are frequently unsuitable for TP and SG evaluation for the same reason.11,13,17 Bloody samples should be handled as above, while relatively transparent samples can be measured without former centrifugation.5 Regarding effusions with suspected FIP, TP values above 35 g/L (>50% globulins) have been reported to attain sensitivity of 100% for FIP diagnosis.24
Biochemical analysis
The biochemical analytes usually evaluated in effusions include levels of pH, GLU, albumins (ALB), globulins (GLOB), triglycerides (TG), lactate dehydrogenase (LDH) and cholesterol (CHOL). These analytes are concurrently determined in the animal patient’s fluid and serum and subsequently compared.
The pH determination of pleural fluids is a valuable prognostic tool for parapneumonic effusions in human medicine.25 It is usually performed by the introduction of a pH-meter sensor in the collected sample or a blood gas analyzer. However, precision in pH values is rarely attained as the sample to be evaluated should be air-free, following collection using a heparinised syringe, and subsequently examined rapidly. As a general rule, pH values decline in exudative effusions as the result of lactic acid produced by the bacterial population.12
Lactate dehydrogenase values in pleural effusions demonstrate the degree of inflammation in the pleural cavity, as LDH is released from damaged or destroyed cells.12,13,25 In humans, high LDH values are usually encountered in exudates.13 Lactate dehydrogenase values have also been determined in feline pleural effusions. Indicatively, in cases of FIP, pleural effusions have LDH values over 300 IU/L, while LDH values in septic exudates usually exceed 200 IU/L. Lactate dehydrogenase levels in the effusion, along with the pleural fluid/serum TP ratio, have been found to attain sensitivity of 100% and 91% and specificity of 100%, respectively, for the differentiation between transudative and exudative effusions in cats.13
The determination of lactate values for the differentiation of septic from non-septic pleural effusions has not been adequately evaluated in dogs and cats. Conversely, the concentration of lactate in feline peritoneal effusions was not considered reliable for the diagnosis of septic effusions.26
Concentrations are usually low in exudates, possibly as a result of the metabolic consumption of GLU from leucocytes, neoplastic cells and/or pleura, as well as their irregular transportation from inflamed pleural membranes.12 Some studies relate the pH values in pleural effusions to their respective GLU concentration.13 In septic exudates, GLU concentration is usually below 1.7 mmol/L, while in neoplastic eff usions it ranges between 0.5 and 4.5 mmol/L.13
Albumin-globulin ratio is mostly used in pleural eff usions with suspected FIP and is considered to possess the highest diagnostic value compared to other tests for FIP.18 As FIP eff usions are typically characterized by high TP concentrations, with GLOB being at least 50%, an ALB-GLOB ratio below 0.81 and especially below 0.4 is suggestive of the disease.5,24 In-house dry chemistry analyzers have been considered to lack precision for ALB determination in feline eff usions in contrast to wet chemistry analysers.22
Triglycerides and CHOL concentrations in pleural effusions are considered the best markers for the diff erentiation of chylous from pseudochylous eff usions (rarely reported in dogs and cats) or for the diagnosis of atypic chylous effusions.5 In particular, chylous effusions have higher triglyceride and lower cholesterol concentrations than those in serum, while the opposite applies to pseudochylous eff usions.5 A CHOL-TRIG ratio <1 is suggestive of chylous effusions.27 It should be noted that pleural effusions due to feline cardiac disease are considered chylous.7
N-terminal pro-B-type natiuretic peptide values have been found to be elevated in both sera and pleural effusions from cats with cardiac disease. However, further studies are needed for the evaluation of this biomarker.28
Microbiological examination
Microorganisms found in pleural effusions are either due to an endogenous infl ammatory process (pyothorax) or iatrogenic contamination.14 In cats, obligate anaerobes and facultative anaerobes such as Pasteurella spp. are most commonly isolated.3 In a study of cats with pyothorax, 89% of samples yielded obligate anaerobes, while 44% yielded a mixture of obligate anaerobes and facultative bacteria.29 Regarding fungi, Histoplasma capsulatum and Cryptococcus neoformans are mostly considered to be related to pleural eff usion formation in cats,30 although they do not constitute common entities for veterinary practice in Greece.
Chylous samples, in which bacterial growth normally is not facilitated because of the presence of fatty acids, might also be submitted for microbiological examination, especially when repeated thoracocenteses have been perfomed.3 In a study of 37 cats with chylothorax, microbiological cultures were positive in 13.5% of cases (iatrogenic pyothorax).31 It should be noted that previous antibiotic or antifungal therapies administered must always be considered when submitting samples for microbiological examination.
> Other tests
Additional diagnostic procedures available for pleural eff usion investigation not only include plain methods like the Rivalta test but also advanced laboratory techniques such as histopathology, immunocytochemistry, immunofl uorescence, reverse transcription-polymerase chain reaction/ enzyme linked immunosorbent assay (RT-PCR/ ELISA) and electrophoresis. The above tests are performed especially in cats with suspected FIP in order to establish diagnosis.
Rivalta’s test
 On a primary basis, the Rivalta’s test has been used for the diff erentiation of transudative from exudative eff usions. The test has been most commonly used for the diagnosis of FIP eff usions, for which it has been reported to yield specifi city of 80% and sensitivity of 98%.18 However, Fischer et al. have recently reported 91% sensitivity, 66% specifi city and a positive predictive value (PPV) of 58% for the Rivalta’s test.32 Positive results are linked to high concentrations of protein, fi brin and infl ammatory mediators, while false positive results may arise in patients with bacterial peritonitis or lymphoma.18 The procedure is described as follows: 5 ml of distilled water is placed in a sample tube along with 1 drop of acetic acid (98% v/v). Subsequently, 1 drop of the effusion under study is placed on the upper surface of the solution. The test is characterized as “positive” when the drop remains on the solution surface or sinks slowly to the bottom of the tube, or “negative” when the drop dissipates in the solution (Figure 1).
On a primary basis, the Rivalta’s test has been used for the diff erentiation of transudative from exudative eff usions. The test has been most commonly used for the diagnosis of FIP eff usions, for which it has been reported to yield specifi city of 80% and sensitivity of 98%.18 However, Fischer et al. have recently reported 91% sensitivity, 66% specifi city and a positive predictive value (PPV) of 58% for the Rivalta’s test.32 Positive results are linked to high concentrations of protein, fi brin and infl ammatory mediators, while false positive results may arise in patients with bacterial peritonitis or lymphoma.18 The procedure is described as follows: 5 ml of distilled water is placed in a sample tube along with 1 drop of acetic acid (98% v/v). Subsequently, 1 drop of the effusion under study is placed on the upper surface of the solution. The test is characterized as “positive” when the drop remains on the solution surface or sinks slowly to the bottom of the tube, or “negative” when the drop dissipates in the solution (Figure 1).
Direct immunofluorescence
Direct immunofl uorescence (DIF) is performed either on cytological or biopsy preparations for the detection of Feline Coronavirus (FCoV) antigen. The method has been reported to have sensitivity of 100% and specifi city of 71.4% in eff usion samples.32 In biopsy samples, the method possesses sensitivity and specificity 100% for FIP diagnosis. However, it attains low negative predictive value (NPV).33
Electrophoresis
Electrophoresis is mostly used in effusions highly suggestive of FIP, when a2- and γ- globulins concentrations rise. This method has been reported to have 100% positive predictive value (PPV) when γ-globulin content was >32%.34 Lipoprotein electrophoresis has also been performed in pleural effusions from cats for the diff erentiation of chylous from non-chylous eff usions.35
> Cytological evaluation
Cytology attains sensitivity of 61% and specificity of 100% in the diagnosis of neoplastic body cavity effusions in cat patients; thus, along with histopathology, it remains a valuable tool for establishing diagnosis.36
Cells types
The cell types that might be encountered in cat pleural effusions are mesothelial cells, RBCs, inflammatory cells such as mature, non- and degenerated or hypersegmented neutrophils, non- and activated macrophages, small lymphocytes and eosinophils, as well as neoplastic cells.5,7,11 Rarely, mast cells may also be encountered.7,11 Apart from cells, other features may also be observed such as bacteria, fungi and plant fibres.
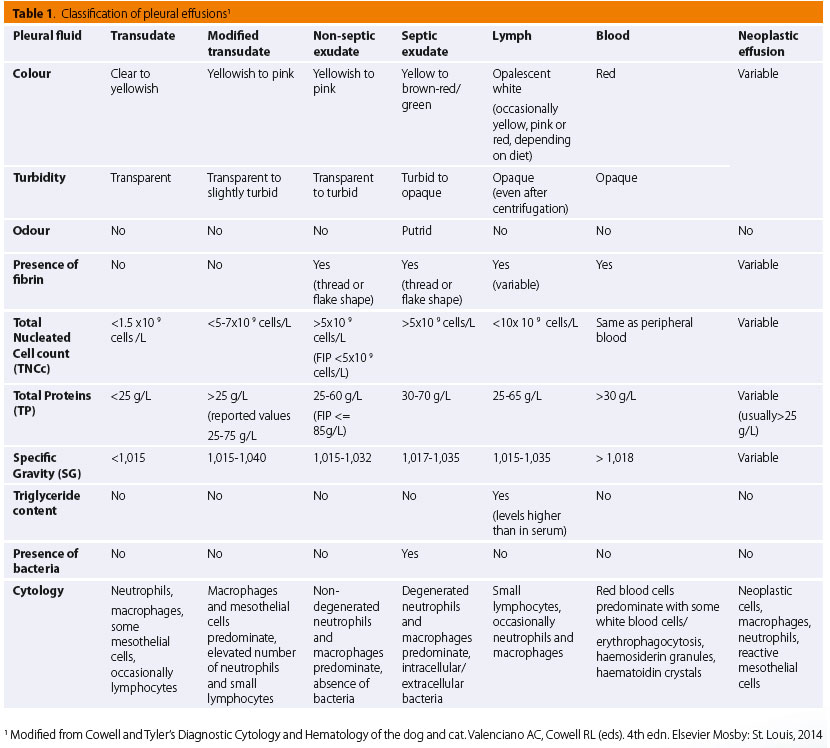
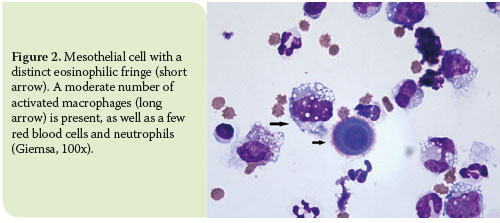
As to mesothelial cells, these normally line the pleural cavities and are either naturally exfoliated in the pleural fluid or dislodged during sample collection.11 Additionally, mesothelial cells are considered to play a role in the infl ammatory process by means of cytokine production and antigen presentation.5 In terms of morphology, mesothelial cells are large cells with a round to oval nucleus which is typically centrally located and exhibits a uniformly-distributed-chromatin pattern, and basophilic cytoplasm. Occasionally, an eosinophilic fringe may be present peripherally.8 Mesothelial cells can be found either as single cells or in sheets (Figure 2-3).8
Eosinophilic pleural effusions in cat patients have previously been linked to pneumothorax, feline immunodeficiency virus infection, visceral mast cell tumours, hypereosinophilic syndrome and pulmonary parasitic infections.37,38,39,40 Eosinophil percentages above 10% of TNNC in eff usions may be indicative of a neoplastic disease.
Transudates (true and modified)
 In true transudates, cytological findings are characterized as non-typical and usually involve low counts of mature, non-degenerated neutrophils, mesothelial cells, macrophages at various stages of activation and small lymphocytes.5,11
In true transudates, cytological findings are characterized as non-typical and usually involve low counts of mature, non-degenerated neutrophils, mesothelial cells, macrophages at various stages of activation and small lymphocytes.5,11
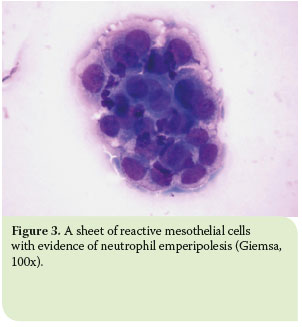
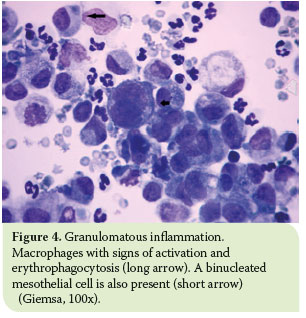
The most distinctive cytological feature of modified transudates is reactive mesothelial cells.11 Due to effusion chronicity, mesothelial cells usually tend to react and thus become binucleated, with multiple, distinct nucleoli, and increased phagocytic activity.19 Moreover, high counts of non-degenerated neutrophils are commonly observed as well as activated macrophages (Figure 4).7,11
Exudates (septic and non-septic)
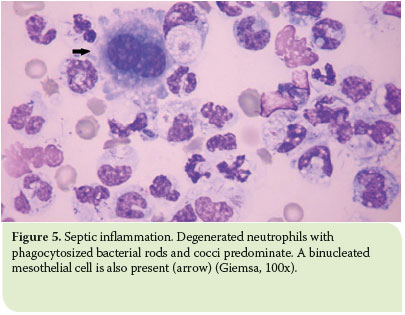 Septic exudates are typically characterised by the predomination of degenerated neutrophils and the presence of intracellular and/or extracellular bacteria. Against the smear background, eosinophilic proteinaceous material is frequently observed. Degenerated neutrophils have been subjected to hydropic degeneration due to their exposure to bacterial toxins, and exhibit a swollen nucleus with less noticeable lobulation which is stained as eosinophilic with Romanowsky-type stains.7 In the presence of degenerated neutrophils, the smear should be extensively examined for bacteria, whilst when degenerated neutrophils are not observed, a bacterial infection should not be excluded as toxin quality and quantity vary according to the species and number of bacteria present.7
Septic exudates are typically characterised by the predomination of degenerated neutrophils and the presence of intracellular and/or extracellular bacteria. Against the smear background, eosinophilic proteinaceous material is frequently observed. Degenerated neutrophils have been subjected to hydropic degeneration due to their exposure to bacterial toxins, and exhibit a swollen nucleus with less noticeable lobulation which is stained as eosinophilic with Romanowsky-type stains.7 In the presence of degenerated neutrophils, the smear should be extensively examined for bacteria, whilst when degenerated neutrophils are not observed, a bacterial infection should not be excluded as toxin quality and quantity vary according to the species and number of bacteria present.7
Nonetheless, the key to cytological diagnosis of septic exudates is the observation of intracellular bacteria, while observation of extracellular bacteria might be due to a contaminated stain. Bacteria usually appear light pink to deeply basophilic when a Romanowsky-type stain is used and are most commonly found phagocytised by neutrophils and sometimes by macrophages (Figure 5).5
In non-septic exudates, non-degenerated neutrophils mainly predominate, while bacteria are not observed.5,8 Activated macrophages may also be present, as well as a low number of small lymphocytes.5,8
Chylous and pseudochylous effusions
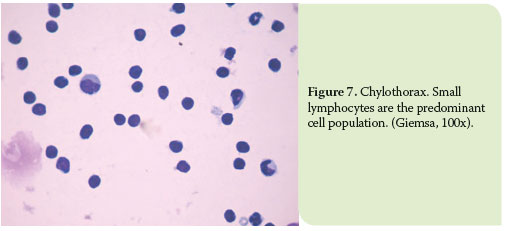
In chylous effusions, small lymphocytes usually predominate in the overall cell population, while lipid droplets might be found in the background.5,8 However, as the effusion becomes chronic, pleura may exhibit signs of inflammation and thus, the predominant cell types are mature, nondegenerated neutrophils and/or macrophages (mostly activated, containing cytoplasmic vacuoles, which are related to lipids).7 Moreover, reactive mesothelial cells may also be reported, due to the irritation of pleura, as well as reactive lymphocytes (Figure 6-7).5
On the other hand, in pseudochylous effusions, the cytology is variable depending on the primary cause of the effusion. Hence, in pseudochylous effusions of neoplastic origin, neoplastic cells prevail, while in pseudochylous effusions of inflammatory origin, non-degenerated neutrophils and /or macrophages predominate.5
Haemorrhagic effusions
 In haemorrhagic effusions, blood cells typically prevail as they exist in the peripheral blood of the cat patient. Macrophages and mesothelial cells may occasionally be observed. However, the presence of platelets varies, depending on whether there is chronic haemorrhage or peripheral contamination and/or per-acute haemorrhage.5,8 In the fi rst case, platelets are usually absent as they tend to form aggregations, become degranulated and thus, rapidly disappear.5,8 On the other hand, in cases of peripheral blood contamination and/or per-acute haemorrhage, platelets are usually present in the smear.5,8
In haemorrhagic effusions, blood cells typically prevail as they exist in the peripheral blood of the cat patient. Macrophages and mesothelial cells may occasionally be observed. However, the presence of platelets varies, depending on whether there is chronic haemorrhage or peripheral contamination and/or per-acute haemorrhage.5,8 In the fi rst case, platelets are usually absent as they tend to form aggregations, become degranulated and thus, rapidly disappear.5,8 On the other hand, in cases of peripheral blood contamination and/or per-acute haemorrhage, platelets are usually present in the smear.5,8
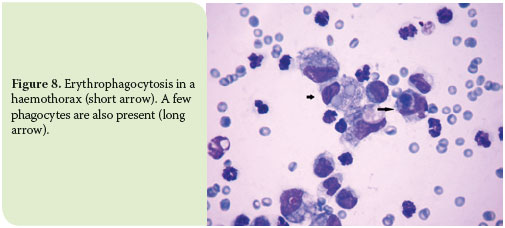
Chronic haemorrhages are also accompanied by the remarkable presence of activated erythrophages and signs of haemoglobin decomposition. The latter is marked by the presence of black-green granules (haemosiderin), mainly in the cytoplasm of macrophages and also in the background, as well as by the presence of rhomboid, yellow to orange crystals (haematoidin) in the cytoplasm of macrophages, especially when the haemorrhage has progressed in chronicity (Figure 8).5
Neoplastic effusions
The diagnostic value of cytology when neoplastic effusions are examined basically depends on the propensity of the neoplasm for exfoliation. In particular, neoplasms of epithelial origin, as well as round-cell tumours, tend to become easily exfoliated into body eff usions, thus yielding samples of moderate to high cellularity.5 Signs of co-existent infl ammation may also be present.7,8
Intrathoracic tumours, whether primary or metastatic, are commonly involved in pleural eff usion formation in cats.47 Primary tumours in cats involve mesotheliomas and pulmonary tumours such as bronchial adenoma/adenocarcinoma and bronchioalveolear carcinomas. Mediastinal lymphomas are more often encountered from round-cell tumours, whereas sarcomas, both metastatic and primary, are rarely found in pleural cavities.7,8
Neoplastic effusions usually fall into the modified transudate or exudate categories, although chylous or haemorrhagic effusions may also occur.5,7
 The most distinctive feature about carcinomas is that the epithelial cells are typically presented in clusters or sheets, while acinar patterns may sometimes be noted (adenocarcinomas).7,8 Neoplastic epithelial cells mainly exhibit anisokaryosis, multinucleation, high nuclear-cytoplasmic ratio, coarse nuclear chromatin pattern, multiple and prominent nucleoli, with variable appearance and abnormal mitoses.7 Basophilia and vacuolation of the cytoplasm may also be reported. In mesotheliomas, cells are characterized by the aforementioned criteria of malignancy. Common misinterpretations occur with cytology between hyperplastic or reactive mesothelial cells, neoplastic mesothelial cells and carcinomas, as the first tend to have features that assimilate malignancy.5,7,8
The most distinctive feature about carcinomas is that the epithelial cells are typically presented in clusters or sheets, while acinar patterns may sometimes be noted (adenocarcinomas).7,8 Neoplastic epithelial cells mainly exhibit anisokaryosis, multinucleation, high nuclear-cytoplasmic ratio, coarse nuclear chromatin pattern, multiple and prominent nucleoli, with variable appearance and abnormal mitoses.7 Basophilia and vacuolation of the cytoplasm may also be reported. In mesotheliomas, cells are characterized by the aforementioned criteria of malignancy. Common misinterpretations occur with cytology between hyperplastic or reactive mesothelial cells, neoplastic mesothelial cells and carcinomas, as the first tend to have features that assimilate malignancy.5,7,8
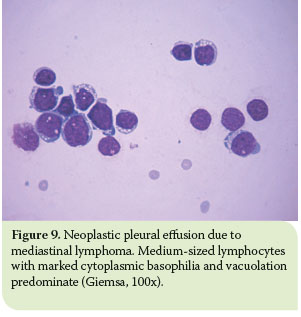
In terms of mediastinal lymphomas, medium-sized lymphocytes and lymphoblasts are predominant in the cell population (usually, over 50% of the population).7,8 These cells may exhibit multiple, irregularly-shaped nucleoli and cytoplasmic basophilia and vacuolation. Moreover, several mitotic fi gures and lymphoglandular bodies might be present.8 Scarcely, small cell lymphomas occur in which small lymphocytes predominate (Figure 9).8,11,14
> Conclusion
Whether performed in-clinic or by an external laboratory, the clinicopathological investigation of feline pleural effusions is an essential component of routine diagnosis. The categorisation of eff usions into transudates, modifi ed transudates and exudates, as well as into haemorrhagic and chylous eff usions, is vital to the understanding of the aetiopathogenesis underlining their formation. Once the latter is established, a more focused therapeutic management is made possible.
> References
1. Nelson LO, Sellon RK. Pulmonary parenchymal diseases. In: Textbook of Veterinary Internal Medicine. Ettinger SJ, Feldman EC (eds). 6th edn. Saunders Elsevier: St. Louis, 2004, pp. 1239-1266.
2. Fossum TW, Miller MW, Rogers KS, Bonagura JD, Meurs KM. Chylothorax associated with right-sided heart failure in fi ve cats. J Am Vet Med Assoc 1994, 204(1): 84-89.
3. Moore LE, Biller DS. Mediastinal disease. In: Textbook of veterinary internal medicine. Ettinger SJ, Feldman EC (eds). 6th edn. Saunders Elsevier: St. Louis, 2004, pp. 1266-1283.
4. Bexfi eld N, Lee K. Thoracocentesis. In: BSAVA Guide in Procedures in Small Animal Practice. Bexfi eld N, Lee K (eds). British Small Animal Veterinary Association: Gloucester, 2011, pp. 195-197.
5. Papasouliotis K, Dewhurst E. Body cavity eff usions. In: BSAVA manual of canine and feline clinical pathology. Villiers E, Blackwood L (eds). 2nd edn. BSAVA: Gloucester, 2005, pp. 340-354.
6. Reece WO. The Respiratory System. In: Functional anatomy and physiology of domestic animals. Reece WO (ed). 4th edn. Willey-Blackwell: Iowa, 2009, pp. 269-311.
7. Valenciano AC, Arndt TP, Rizzi TE. Eff usions: Abdominal, Thoracic and Pericardial. In: Cowell and Tyler’s Diagnostic Cytology and Hematology of the dog and cat. Valenciano AC, Cowell RL (eds). 4th edn. Elsevier Mosby: St. Louis, 2014, pp. 244-265.
8. Rebar AH, Thompson CA. Body Cavity Fluids. In: Canine and feline cytology. Raskin RE, Meyer DJ (eds). edn. Saunders Elsevier: St. Louis, 2009, pp. 171-190.
9. Stillion JR, Letendre JA. A clinical review of the pathophysiology, diagnosis and treatment of pyothorax in dogs and cats. J Vet Emerg Crit Care 2015, 25(3): 113-129.
10. Wustefeld-Janssens BG, Loureiro JF, Dukes-McEwan, German AJ, Burrow RD. Biliothorax in a Siamese cat. J Fel Med Surg 2011, 13(12): 984-987.
11. Rebar AH, DeNicola DB. Cytology of body fl uids. In: Congress proceedings of the North American Veterinary Conference. Orlando, Florida, 2007, pp. 248-252.
12. Light RW. Pleural eff usion. In: Textbook of Respiratory Medicine, Murray JF, Nadel JA (eds). 2nd edn. WB Saunders: Philadelphia, 1994, pp. 2164-2192.
13. Zoia A, Slater LA, Heller J, Connolly DJ, Church DB. A new approach to pleural eff usion in cats: markers for distinguishing transudates from exudates. J Feline Med Surg 2009, 11(10): 847-885.
14. De Nicola DB. Feline thoracic and abdominal eff usion evaluation. Common presentations. In: Congress proceedings of the international congress of the Italian Association of Companion Animal Veterinarians. Rimini, Italy, 2008, pp. 139-140.
15. Baker R, Lumsden. JH. Pleural and peritoneal fl uids. In: Color atlas of cytology of dog and cat. Baker R, Lumsden JH (eds). Mosby: St. Louis, 2000, pp. 159-176.
16. Smith RIE. The cat with a cloudy eye. In: Problem-based feline medicine. Rand J (ed). 1st edn. Saunders Elsevier: St. Louis, 2006, pp. 1254- 1277.
17. Kerr MG. Non-blood body fl uids. In: Veterinary laboratory medicine. Kerr MG (ed). 2nd edn. Blackwell Science Ltd: Oxford, 2002, pp. 169-180.
18. Hartmann K, Binder C, Hirschberger J, Cole D, Reinacher M, Schroo S, Frost J, Egberink H, Lutz H, Hermanns W. Comparison of diff erent tests to diagnose feline infectious peritonitis. J Vet Intern Med 2003, 17(6): 781-790.
19. Giannasi C, Brown A and Skeldon N. Evaluation of HemoCue WBC as a bedside analyser in chacterising abdominal eff usions. In: BSAVA Congress Scientifi c Proceedings Abstracts. Quedgeley, Gloucester, 2013, pp. 558.
20. Welles EG, Oller E, Spangler EA, Suddeth J. Validation of an inoffice automated haematology instrument, the Heska CBC-Diff , for total nucleated cell counts in body cavity eff usions and comparison of diff erential cell counts with manual observations from preapared smears. In: ASVCP Annual Meeting Abstracts. Nashville, TN, 2011, p. 597.
21. Stockham SL, Scott MA. Cavitary eff usions. In: Fundamentals of Veterinary Clinical Pathology. Stockham SL, Scott MA (eds). 2nd edition. Blackwell Publishing: Iowa, 2008, pp. 831-868.
22. Papasouliotis K, Murphy K, Dodkin S, Torrance AG. Use of the Vettest 8008 and Refractometry for Determination of Total Protein, Albumin and Globulin Concentrations in Feline Eff usions. Vet Clin Path 2002, 31(4): 162- 166.
23. Braun JP, Guelfi JF, Pages JP. Comparison of four methods for determination of total protein concentrations in pleural and peritoneal fl uid from dogs. Am J Vet Res 2001, 62(3): 294-296.
24. Sparkes AH, Gruff ydd-Jones TJ, Harbour DA. An appraisal of the value of laboratory tests in the diagnosis of feline infectious peritonitis. J Am Anim Hosp Assoc 1994, 30(4): 345-350.
25. Herrtage ME. Respiratory disorders. In: Clinical medicine of dog and cat. Schaer M (ed). 2nd edn. Manson Publishing: 2003, pp. 183-188.
26. Bonczynski JJ, Ludwig LL, Barton LJ, Loar A, Peterson ME. Comparison of peritoneal fl uid and peripheral blood pH, bicarbonate, glucose, and lactate concentration as a diagnostic tool for septic peritonitis in dogs and cats. Vet Surg 2003, 32(2): 161-166.
27. Fossum TW, Jacobs RM, Birchard SJ. Evaluation of cholesterol and triglyceride concentrations in diff erentiating chylous and non-chylous pleural eff usions in dogs and cats. J Am Vet Med Assoc 1986, 188(1): 49-51.
28. Hassdenteufel E, Henrich E, Hildenbrandt N, Stosic A, Schneider M. Assessment of circulating N-terminal pro B-type natriuretic peptide concentration to diff erentiate between cardiac from noncardiac causes of pleural eff usions in cats. J Vet Emerg Crit Care 2013, 23(4): 416-422.
29. Love DN, Jones RF, Bailey M, Johnson RS, Gamble N. Isolation and characterization of bacteria from pyothorax (empyaemia) in cats. Veterinary Microbilogy 1982, 7: 455-461.
30. Sauve V. Pyothorax in cats: a Review. Full Circle Forum 2011, 4: 1-2.
31. Forester SD, Troy GC, Fossum TW. Pleural eff usions: pathophysiology and diagnostic considerations. Comp Cont Educ 1988, 10: 121-137.
32. Fischer Y, Sauter-Louis C, Hartmann K. Diagnostic accuracy of the Rivalta test for feline infectious peritonitis. Vet Clin Path 2012, 41(4): 558- 567.
33. Paltrinieri S, Parodi Cammarata M, Camarata G. In vivo diagnosis of feline infectious peritonitis by comparison of protein content, cytology and direct immunofl uorescence test on peritoneal and pleural eff usions. J Vet Diagn Invest 1999, 11(4): 358-361.
34. Shelly SM, Scarlett-Kranz J, Blue JT. Protein electrophoresis on eff usions from cats as a diagnostic test for feline infectious peritonitis. J Am Anim Hosp Assoc 1988, 24(5): 495-500.
35. Waddle JR, Giger U. Lipoprotein Electrophoresis Diff erentiation of Chylous and Nonchylous Pleural Eff usions in Dogs and Cats and Its Correlation with Pleural Eff usion Triglyceride Concentration. Vet Clin Path 2009, 19(3): 80-85.
36. Hirshberger J, Koch S. Validation of the determination of the activity of adenosine deaminase in the body eff usions of cats. Res Vet Sci 1995, 59(3): 226-229.
37. Miller BH, Roudebush P, Ward HG. Pleural eff usion as a sequela to aelurostrongylosis in a cat. J Am Vet Med Assoc 1984, 185(5): 556-557.
38. Fossum TW, Wellman M, Relford RL, Slater MR. Eosinophilic pleural or peritoneal eff usions in dogs and cats: 14 cases (1986-1992). J Am Vet Med Assoc 1993, 202(11): 1873-1876.
39. Peaston AE, Griff ey SM. Visceral mast cell tumour with eosinophilia and eosinophilic peritoneal and pleural eff usions in a cat. Aust Vet J 1994, 71(7): 215-217.
40. Saxon B, Hendrick M, Waddle JR. Restrictive cardiomyopathy in a cat with hypereosinophilic syndrome. Can Vet J 1991, 32(6): 367–369.



