> Abstract
Leukogram includes total and differential white blood cell counts, as well as leukocyte morphology. Appropriate blood sampling and handling are essential for the validity of the leukogram interpretation. Leukocytosis and leukopenia are associated with physiologic or pathologic conditions. From a clinical point of view, neutrophilia, neutropenia, lymphocytosis, lymphopenia, monocytosis and eosinophilia are considered the most important alterations in leukocyte numbers. Neutrophilia and monocytosis usually accompany infl ammatory diseases, but they are also observed in excess of steroids. Additionally, neutrophilia can be physiologic, as well as, lymphocytosis, which is further associated with chronic infl ammatory diseases. Eosinophilia typically occurs in hypersensitivity reactions and parasitisms. Neutropenia is mainly related to infectious diseases, while lymphopenia is usually steroid-induced or related to acute infectious diseases. Monocytopenia, eosinopenia, basophilia and basopenia have limited diagnostic signifi cance.Common morphologic changes of the leukocytes include the presence of immature, hypersegmented or toxic neutrophils, as well as reactive lymphocytes and various leukocyte inclusions. These may be of infectious origin, such as morulae of Ehrlichia canis and Ehrlichia ewingii or of non-infectious origin, such as siderotic inclusions.
> Introduction
Leukogram is part of the complete blood count (CBC) and includes total and differential white blood cell (WBC) counts and WBC morphology evaluated during the microscopic of stained blood smears (Table 1). Appropriate blood collection, sample handling and storage are essential in order to maximize the reliability of the results. Overnight fasting should precede blood collection; the animal must not be under stress and the forma- tion of platelet clumps should be avoided.1 Blood smear preparation should be done as soon as possible and certainly within 2-3 hours after blood collection, while CBC should be completed within 24 hours, provided that blood into the EDTA tube has been stored in the refrigerator.1 Finally, blood smears are stained with Romanowsky-type stains (e.g. Giemsa, Wright, Diff -Quik) for routine examination.
> Neutrophilia and neutropenia
Neutrophilia is defi ned as the increase in circulating neutrophils and is generally characterized as physiologic, steroid-induced or associated with infl ammation and neoplasia. Physiologic neutrophilia (also called pseudoneutrophilia) is the result of the endogenous release or exogenous administration of catecholamines.2 Physiologic neutrophilia is transient, as it occurs within minutes of the stimulus appearance and generally resolves within 30 minutes.3 It is characterized as mature and mild to moderate, and it mainly concerns young animals and especially cats.4,5 Steroid-induced neutrophilia could be of endogenous (severe stress or hyperadrenocorticism) or of exogenous (administration of glucocorticoids) origin.2,4 It is mature, mild to moderate occurs within 4-8 hours after the release or administration of glucocorticoids and resolves within 24 hours.2,4 However, in chronic therapy with glucocorticoids, neutrophilia persists for some days, but it is of lesser degree.6 Apart from neutrophilia, glucocorticoids have multiple eff ects on leukogram (see lymphopenia, monocytosis, eosinopenia). Inflammation is a common cause of neutrophilia. Depending on many factors, such as the underlying etiology, duration and bone marrow responsiveness, neutrophilic leukocytosis varies on severity and can be seen concurrently with left or right shift, lymphopenia or lymphocytosis, eosinophilia or eosinopenia and monocytosis.7 The etiology of infl ammatory neutrophilia includes many infectious (most commonly bacterial) and non-infectious (most commonly immune-mediated) diseases of internal or subcutaneous tissues, whereas infl ammation of the central nervous system, intestine, lower urinary tract and superfi cial cutaneous tissue may not induce neutrophilia.2,4,6 Specific inflammatory diseases (e.g. peritonitis, pyothorax, pyometra) are associated with leukemoid response. Leukemoid response or reaction is defi ned as an extreme infl ammatory “leukemia-like” leukocytosis.2 Typically, circulating neutrophils are 50.000-100.000/μL and severe left shift is usually observed.4 Neoplasia-associated neutrophilic leukocytosis can be seen in the rare case of chronic granulocytic leukemia or as a paraneoplastic syndrome that accompanies benign or malignant neoplasms, such as rectal adenomatous polyps or renal tubular carcinomas, respectively.8,9 In dogs, mature neutrophilia may also be observed during pregnancy.6
Neutropenia is defi ned as the decrease in circulating neutrophils and is less frequent than neutrophilia. Infectious diseases, such as parvoviral enteritis and ehrlichiosis in dogs and panleukopenia, feline leukemia virus (FeLV) and feline immunodefi ciency virus (FIV) infections in cats, induce neutropenia through bone marrow hypoplasia/aplasia.4-6 Additionally, overwhelming bacterial infections, endotoxemia and septicemia may also be responsible for neutropenia in both dogs and cats by increasing the margination or emigration of neutrophils.2 Myelophthisis, due to primary or metastatic neoplasms of the bone marrow and myelodysplasia, usually seen in FeLV positive cats, are causative factors of neutropenia that usually persists and may be accompanied by other cytopenias.6,7 The repeated or prolonged administration of specifi c drugs has also been reported to cause neutropenia due to bone marrow suppression. Estrogen of exogenous or endogenous (e.g. Sertoli cell tumor) origin, after initial neutrophilia, and chemotherapeutic agents, such as doxorubicin, cyclophosphamide and vincristine, are related to predictable toxicity, while cephalosporins, phenylbutazone, griseofulvin are examples of drugs that are related to idiosyncratic toxicity.2 Radiation therapy has also been associated with neutropenia due to the destruction of bone marrow hematopoietic cells.2
> Eosinophilia and eosinopenia
Eosinophilia is defi ned as the increase in circulat- ing eosinophils. Parasitism is the most common cause of eosinophilia.10 Both ectoparasites (e.g. fleas and ticks) and endoparasites (e.g. helminthes) are responsible for the increase in the number of eosinophils.10 Moreover, parasites that invade the tissues, including those which migrate through them, induce greater eosinophilia.6 Hypersensitivity disorders, such as allergic dermatitis and food or drug allergies, typically result in migration of eosinophils to the aff ected tissue; thus, initial eosinopenia is followed within a few days by eosinophilia due to bone marrow response.6 Apart from hypersensitivity disorders, other infl ammatory agents acting in tissues rich in mast cells (the respiratory system, alimentary system, genitourinary system and skin) have been correlated with increased numbers of circulating eosinophils.6,7 Eosinophilia has also been associated with idiopathic eosinophilic conditions (e.g. hypereosinophilic syndrome in cats), as well as with neoplasms, either as a paraneoplastic syndrome (e.g. mast cell tumors) or in rare cases of chronic granulocytic leukemia.7,11,12
Eosinopenia is defined as the decrease in circulating eosinophils. Eosinopenia is of little diagnostic significance and is mainly the result of high glucocorticoids concentration.
> Basophilia and basopenia
Basophilia is defi ned as the increase in the circulating basophils, although to be clinically signifi cant, it should be of a great degree or of extended duration.2 An increase in basophil numbers typically occurs along with eosinophilia in cases of parasitism, hypersensitivity disorders and neoplasia and is rare in clinical practice, while its most common cause by far, is dirofi lariasis.4,5,13
Basopenia is defined as the decrease in circulating basophils, but practically it is undetectable and of no diagnostic significance.
> Lymphocytosis and lymphopenia
Lymphocytosis is defi ned as the increase in circulating lymphocytes. Lymphocytosis is associated mostly with acute stress response (physiologic lymphocytosis), chronic infl ammation, neoplasia and hypoadrenocorticism. Physiologic lymphocytosis is an eff ect of endogenously released or exogenously administered catecholamines and is observed concurrently with neutrophilia. Chronic inflammatory lymphocytosis is part of the hyperplastic lymphoid response to chronic antigenic or cytokine stimulation seen in many bacterial, viral, fungal and protozoal infections.2,14,15 Ehrlichiosis, leishmaniosis, babesiosis and FeLV infection are some examples that are frequently seen in clinical practice.14-17 It is usually of mild to moderate degree and is commonly accompanied by neutrophilia and/or monocytosis and occasionally by eosinophilia and/or basophilia.2 Chronic lymphocytic leukemia/small cell lymphoma stage V is associated with lymphocytosis, which can be marked with or without the evidence of atypical lymphocytes in peripheral blood.18 Both dogs and cats suff ering from hypoadrenocorticism may show lymphocytosis due to the lack of glucocorticoids.19 Finally, it should be taken into consideration that puppies and kittens normally have higher number of circulating lymphocytes than adults until the age of 2 and 4-5 months, respectively.2,6
Lymphopenia is defi ned as the decrease in circulating lymphocytes. It can be steroid-induced, acute inflammatory and due to limited production or loss of lymph. Steroid-induced lymphopenia due to exogenous or endogenous glucocorticoids is the result of the shift of lymphocytes from the circulating pool. In general, the severity and duration of lymphopenia are proportional to the glucocorticoids dose and duration of their increased concentration.2 Acute infl ammatory lymphopenia is typically accompanied by neutrophilia or neutropenia; in such cases, the resolution of lymphopenia is believed to be a good prognostic factor.2 The etiology includes the early stages of many acute viral infections (e.g. canine parvoviral enteritis, canine distemper, FeLV infection) and acute, severe or overwhelming, systemic bacterial infections.2,3,6 Lymphopenia can also be the result of either limited production due to lymphoid hypoplasia or aplasia, or loss of lymphocyte-rich lymph, as seen in chylothorax and proteinlosing enteropathies.4-5
> Monocytosis and monocytopenia
Monocytosis is defi ned as the increase in circulating monocytes. Monocytosis is usually seen concurrently with steroid-induced neutrophilia in dogs, as well as with infl ammatory neutrophilia.18 Marked monocytosis (when there are not many abnormal or atypical monocytes) is also a feature of monocytic or myelomonocytic leukemias.2 Monocytosis along with lymphopenia and eosinopenia may develop with age.6
Monocytopenia is defi ned as the decrease in circulating monocytes. In health, monocyte numbers are very low and, practically, monocytopenia has no diagnostic signifi cance.
> Mastocytemia
Mastocytemia is defi ned as the presence of mast cells in peripheral blood. One mast cell in the blood or buffy coat smears can set the diagnosis of mastocytemia, as mast cells are not found in blood of healthy dogs and cats.2 The etiology of mastocytemia in dogs includes infl ammatory diseases, regenerative anemias, trauma and neoplasms (mast cell tumor and other neoplasms).20,21 On the other hand, circulating mast cells are very infrequent finding in cats and are strongly associated with visceral mast cell tumor.22
> Leukocyte morphology
The evaluation of blood smears stained with a Romanowsky-type stain is an essential part of the CBC, especially when automated blood counts reveal leukocytosis, leukopenia or abnormalities in the diff erential WBC count. For this reason, a manual diff erential leukocyte count is necessary in order to determine the relative numbers of WBCs in the blood.23 Abnormal fi ndings during the microscopic examination of the blood concern the nucleus (e.g. hyposegmentation, hypersegmentation) and/or cytoplasm (e.g. toxic changes, inclusions) of WBCs. The morphologic changes of WBCs, which will be described in the following part, are left and right shift, toxic neutrophils, reactive lymphocytes and WBC inclusions.
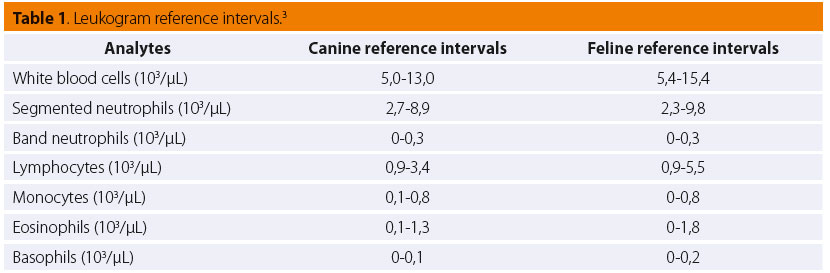
Left shift (or shift to the left) is defi ned as an increase in concentration of circulating non-segmented neutrophils above 1,000/μL or 10% of total WBC count in the presence of leukopenia.24 Band neutrophils are mostly observed (Figure 1), but if the demand for neutrophils in tissues is so great, more immature cells, such as metamyelocytes, myelocytes and even promyelocytes (rarely though) may be present in the blood.18 A left shift is often associated with an acute infl ammatory process and its severity is proportional to the degree of the immaturity of neutrophils and concentration of nonsegmented neutrophils in the blood.2 Left shift can be either regenerative or degenerative. Regenerative left shift is characterized by neutrophilia, more segmented neutrophils than non-segmented, and is considered as an appropriate response, whereas degenerative left shift is characterized by normal or slightly increased neutrophil numbers in the blood, less segmented than non-segmented neutrophils and is considered as an inappropriate response, suggestive of a guarded or poor prognosis.4
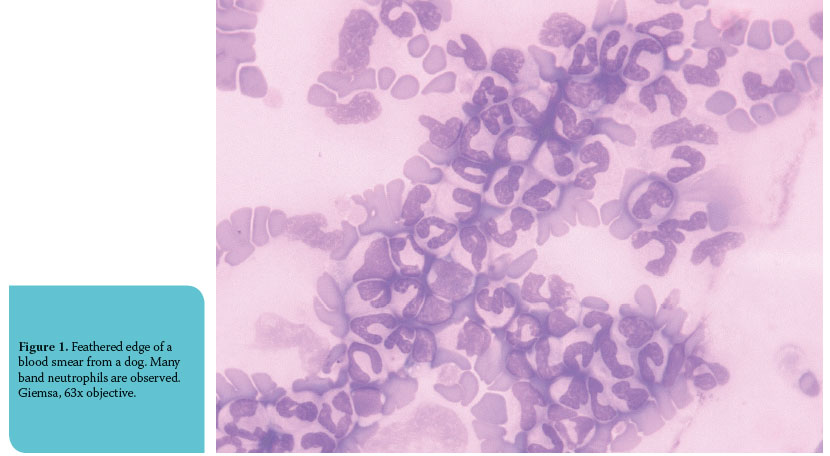
Right shift (or shift to the right) is the increased con- centration of hypersegmented neutrophils (neutrophils with five or more nuclear lobes) in peripheral blood (Figure 2). By far the most frequent cause of right shift is the release or administration of glucocorticoids, which causes a decrease in neutrophil emigration.2 Furthermore, it should be taken into consideration that the delay of blood analysis leads to right shift due to the in vitro aging of the blood cells.
Toxic neutrophils are neutrophils (mature or immature) that have one or more of the following features: foamy cytoplasm, diff use cytoplasmic basophilia, cytoplasmic Döhle bodies (but may be seen in healthy cats), asynchronous nuclear maturation, very large dimensions (giant neutrophils) and toxic granules (primary granules that are present after the promyelocyte stage).2,3 These morphologic changes are developed in the cells of bone marrow; they occur due to expeditious neutropoiesis and represent maturation defects.18 The presence of toxic neutrophils in the blood is generally related to left shift and the more severe the toxic changes are, the poorer the prognosis.16 Toxic neutrophils are usually associated with bacterial or viral infections, immune-mediated hemolytic anemia (IMHA), severe metabolic diseases and certain drugs administration.3,4,24,25
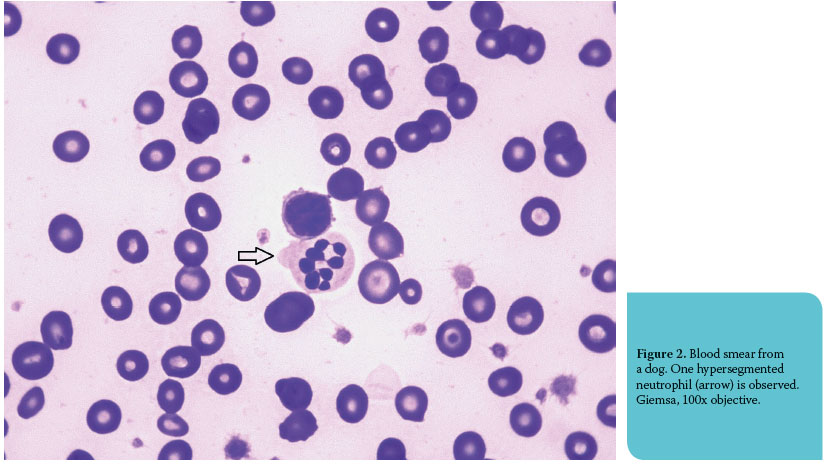
Reactive lymphocytes (plasmacytoid lymphocytes, immunocytes and virocytes) are B-lymphocytes or stimulated T-lymphocytes, which can normally be found in low numbers in peripheral blood (common in young animals), but also in high numbers in infl ammatory diseases (especially in chronic disease). Additionally, the presence of medium or large lymphocytes and mitotic fi gures can be the result of reactive hyperplasia (Figure 3).2
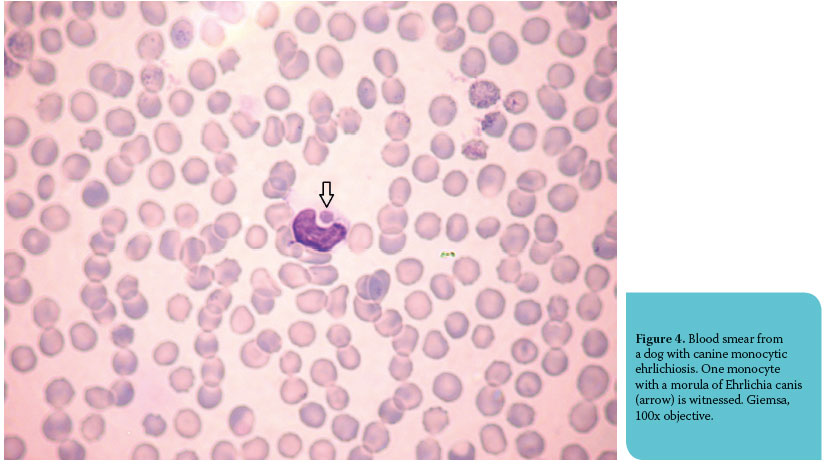
Leukocyte inclusions are either of infectious or noninfectious origin. The first category includes bacterial, viral, protozoal and fungal diseases, whereas the second includes immunological and hereditary diseases aff ecting WBC morphology. Morulae of Ehrlichia canis, Ehrlichia ewingii and Anaplasma phagocytophilum are clusters of organisms that resemble morulae and are stained magenta to blue-grey with Romanowsky-type stains.2,4 They are observed infrequently and only during the acute phase of the disease.3 Ehrlichia canis infects the monocytes (Figure 4) and lymphocytes of dog, Ehrlichia ewingii infects the granulocytes and rarely the monocytes of dog and Anaplasma phagocytophilum can be found in neutrophils and eosinophils.2 Gametocytes of Hepatozoon canis are oval to elliptical, pale blue and are seen in neutrophils and monocytes of dog.2 Canine distemper inclusions are red to pale blue cytoplasmic inclusions that can be found during the viremic stage of the infection in neutrophils, lymphocytes and monocytes.2,3 Bacteria are rarely seen in neutrophils of bacteremic patients, while inclusions of infectious origin that are extremely uncommon fi ndings in blood smears are Leishmania spp. amastigotes, tachyzoites of Toxoplasma gondii, Mycobacterium spp. and yeast phase of Histoplasma capsulatum.2,4 Siderotic inclusions (or hemosiderin granules) can be found, albeit rarely, in monocytes or neutrophils in animals with hemolytic anemias and they are represented by blue-green or yellowbrown pigment on blood smears stained with Romanowsky-type stains.2 The diff erentiation between these inclusions and Döhle bodies relies on staining with Prussian blue (hemosiderin granules stain positively).3 Infrequent fi ndings of the microscopic examination of blood are erythrophagocytosis and lupus erythematosus (LE) cells. The former concerns monocytes and neutrophils and is occasionally ob- served in IMHA, while LE cells are neutrophils that contain pink or pale blue inclusions (on Romanowsky-type stains) which vary in size and represent antigen-antibody complexes.2 Hereditary disorders as- sociated with WBC inclusions aff ect both dogs and cats and are rarely seen in clinical practice. ChediakHigashi syndrome is characterized by large, lightly eosinophilic cytoplasmic granules (Romanowskytype stains) that are contained in granulocytes.25 In hereditary anomaly of neutrophil granulation in Birman cats, neutrophil cytoplasmic granules that are stained deep pink to purple with Romanowskytype stains are seen and resemble the azurophilic granules of promyelocytes.25 May-Hegglin anomaly is characterized by the presence of one to four blue neutrophil cytoplasmic inclusions that resemble Döhle bodies, although they are larger and more prominent.26 Finally, lysosomal storage diseases are associated with the occurrence of cytoplasmic vacuoles or granules in leukocytes.11
> Conclusion
In conclusion, the interpretation of leukogram constitutes a valuable diagnostic tool for the clinician since it contributes to the diagnosis, treatment and prognosis of many diseases and pathologic states of dogs and cats.
> References
1. Weiss D, Tvedten H. The complete blood count and bone marrow examination: general comments and selected techniques. In: Small animal clinical diagnosis by laboratory methods. Willard MD, Tvedten H (ed). 4th edn. Elsevier: St. Louis, 2004, pp. 14-37.
2. Stockham SL, Scott MA. Leukocytes. In: Fundamentals of veterinary clinical pathology. Stockham SL, Scott MA (ed). 2nd edn. Blackwell Publishing: Ames, 2008, pp. 53-106.
3. Harvey JW. Evaluation of leukocytic disorders. In: Veterinary hematology: a diagnostic guide and color atlas. Harvey JW (ed). 1st edn. Elsevier Saunders: St. Louis, 2012, pp. 122-176.
4. Schultze E. Interpretation of canine leukocyte responses. In: Schalm’s veterinary hematology. Weiss DJ, Wardrop KJ (ed). 6th edn. Blackwell Publishing Ltd.: Ames, 2010, pp. 321-334.
5. Valenciano AC, Decker LS, Cowell RL. Interpretation of feline leukocyte disorders. In: Schalm’s veterinary hematology. Weiss DJ, Wardrop KJ (ed). 6th edn. Blackwell Publishing Ltd.: Ames, 2010, pp. 335-344.
6. Bush BM. White blood cells (WBCs). In: Interpretation of laboratory results for small animal clinicians. Bush BM (ed). 1st edn. Blackwell Publishing: Oxford, 1991, pp. 132-195.
7. Blackwood L. Disorders of leucocytes. BSAVA Manual of canine and feline clinical pathology. Villiers E, Blackwood L (ed). 2nd edn. BSAVA: Shurdington, 2005, pp. 58-82.
8. Thompson JP, Christopher MM, Ellison GW, Homer BL, Buchanan BA. Paraneoplastic leukocytosis associated with a rectal adenomatous polyp in a dog. J Am Vet Med Assoc 1992, 201: 737-738.
9. Lappin MR, Latimer KS. Hematuria and extreme neutrophilic leukocytosis in a dog with renal tubular carcinoma. J Am Vet Med Assoc 1988, 192: 1289-1292.
10. Grodecki K. Eosinophilia. In: Manual of canine and feline haematology and transfusion medicine. Day MJ, Mackin A, Littlewood JD (ed). 1st edn. BSAVA: Gloucester, 2000, pp. 131-138.
11. Brockus CW. Leukocyte disorders. In: Textbook of veterinary internal medicine. Ettinger SL, Feldman EC (ed). 6th edn. Saunders Elsevier: St. Louis, 2004, pp. 1937-1943.
12. Lewis MG, Kociba GJ, Rojko JL, Stiff MI, Haberman AB, Velicer LF, Olsen RG. Retroviral-associated eosinophilic leukaemia in the cat. Am J Vet Res 1985, 46: 1066-1070.
13. Mears EA, Raskin RE, Legendre AM. Basophilic leukaemia in a dog. J Vet Int Med 1997, 11: 92-94.
14. Avery AC, Avery PR. Determining the signifi cance of persistent lymphocytosis. Vet Clin North Am Small Anim Pract 2007, 37: 267-282.
15. Gleich S, Hartmann K. Hematology and serum biochemistry of feline immunodefi ciency virus-infected and feline leukaemia virus-infected cats. J Vet Intern Med 2009, 23: 552-558.
16. Weiser MG, Thrall MA, Fulton R, Beck ER, Wise LA, Van Steenhouse JL. Granular lymphocytosis and hyperproteinemia in dogs with chronic ehrlichiosis. J Am Anim Hosp Assoc 1991, 27: 84-88.
17. Zygner W, Gojska O, Rapacka G, Jaros D, Wedrychowicz H. Hematological changes during the course of canine babesiosis caused by large Babesia in domestic dogs in Warsaw (Poland). Vet Parasitol 2007, 145: 146-151.
18. Weiser G. Interpretation of leukocyte responses in disease. In: Veterinary Hematology and Clinical Chemistry. Thrall MA, Weiser G, Allison RW, Campbell TW (ed). 2nd edn. Wiley-Blackwell: Ames, 2012, pp. 127-139.
19. Willard MD, Schall WD, McCaw DE, Nachreiner RF. Canine hypoadrenocorticism: report of 37 cases and review of 39 previously reported cases. J Am Vet Med Assoc 1982, 180: 59-62
20. McManus PM. Frequency and severity of mastocytemia in dogs with and without mast cell tumors: 120 cases (1995-1997). J Am Vet Med Assoc 1999, 215: 355-357.
21. Stockham SL, Basel DL, Schmidt DA. Mastocytemia in dogs with acute infl ammatory diseases. Vet Clin Pathol 1986, 15(1): 16-21.
22. Piviani M, Walton RM, Patel RT. Signifi cance of mastocytemia in cats. Vet Clin Pathol 2013, 42(1): 4-10. 23. Meyer DJ, Harvey JW. Hematology procedures. In: Veterinary Laboratory Medicine - Interpretation and Diagnosis. Meyer DJ, Harvey JW (ed). 3rd edn. Saunders:St. Louis, 2004, pp. 14-26.
24. Raskin RE, Latimer KS, Tvedten H. Leukocyte disorders. In: Small animal clinical diagnosis by laboratory methods. Willard MD, Tvedten H (ed). 4th edn. Elsevier: St. Louis, 2004, pp. 63-91.
25. Aroch I, Klement E, Segev G. Clinical, biochemical, and haematological characteristics, disease prevalence, and prognosis of dogs presenting with neutrophil cytoplasmic toxicity. J Vet Intern Med 2005, 19: 64-73.
26. Flatland B, Fry MM, Baek SJ, Bahn JH, LeBlanc CJ, Dunlap JR, Carroll RC, Kosiba DJ, Millsaps DJ, Schleis SE. May-Hegglin anomaly in a Pug dog. Vet Clin Pathol 2011, 40: 207-214.



