Aetiopathogenesis, diagnosis and current treatment recommendations
> ABSTRACT
Elbow dysplasia is a condition that causes pain and lameness in large and giant breed dogs. Its origins are genetic and when combined with environmental factors, development of the elbow joint becomes abnormal. Originally, elbow osteochondrosis was considered to be the main cause of this condition. Modern studies claim that the condition, in most cases, is caused by various forms of incongruity between articular surfaces of the three joints forming the elbow. Treatment is surgical and should be performed before the development of osteoarthritic lesions in the joint. Multiple surgical techniques to correct this condition are described in the literature. In cases when radiological examination of the elbow joint reveals severe osteoarthritic lesions, the selection of surgical technique depends on lesion localization.
> Introduction
Elbow dysplasia is a hereditary condition, characterized by the abnormal development of the elbow joint. Since 1993, the International Elbow Working Group (IEWG) has defined that hereditary pathological conditions affecting the elbow joint, reportedly referred to as «elbow dysplasia», include the ununited anconeal process (UAP), osteochondritis dissecans of the medial humeral condyle (OCD), medial coronoid disease (MCD) and incongruity between the articular surfaces of the bones forming the elbow joint (IC).1
The condition mainly affects large and giant breed dogs. Over-represented breeds include Bernese Mountain dogs,2 Labrador retrievers,3 Golden retrievers, Rottweilers and German shepherd dogs.4Rapidly-growing male dogs are affected at double the frequency of female dogs;5,6 medium-sized, chondrodystrophic dog breeds (Dachshund, French bulldog) are affected less commonly.4
The main clinical sign of this condition is lameness, which can develop between three and ten months of age. In some dogs, lameness manifests in adulthood (>6 years old), due to MCD, without any history of lameness at a younger age.7 Both forelimbs are affected in 37-50% of cases.8
Due to the fact that MCD, OCD and IC form lesions in the internal anatomical constituents of the elbow joint (medial coronoid process of the ulna, semilunar ulnar notch and medial part of the humeral condyle), and also because these three abnormal conditions may appear in combination, the more generic term «medial compartment disease» (MCoD) has been suggested as a replacement for previous terms.9
> Aetiopathogenesis
The genetic origins of elbow dysplasia have been investigated with several, large-scale epidemiologic papers. Based on the latter, the disease appears to be inherited differently in each dog breed. Moreover, it has been commonly hypothesized that each form of elbow dysplasia is inherited separately from the rest. The conclusion is that elbow dysplasia is caused by several genetic conditions, which disrupt the development of the elbow joint by several pathogenetic mechanisms. Due to the complexity of its hereditary transmission and the role of environmental factors in the development of the disease, it is maintained that the genetic investigation of elbow dysplasia is impossible in the immediate future.10,11,12
Three aetiopathogenic mechanisms have been reported for elbow dysplasia: osteochondritis dissecans, 13,14 elbow incongruity15 and rotatory instability of the radioulnar joint.16,17
These mechanisms result from genetic predilection in combination with secondary predisposing factors, such as a diet of high caloric density and rigorous exercise, and constitute the main causes for the overt manifestation of the disease.9
In current studies, it is maintained that out of the three aetiopathogenic mechanisms, the most likely to cause overt disease is elbow incongruity. In some dogs, osteochondrosis is an important cause. The mechanism for distal radioulnar joint rotatory instability is currently investigated.
Α. Οsteochondrosis
According to the standard theory, osteochondrosis is responsible for the formation of lesions in the medial coronoid process, the humeral condyle and the growth plate of the anconeal process.13,18
In normal animals, the cartilagenous medial coronoid process undergoes ossification from its base to its peak. It has been proven that although it lacks a separate ossification centre, it has the same ossification centre as the ulnar diaphysis. The ossification process is complete by the age of 5-5½ months. If the endochondral ossification of the cartilagenous medial coronoid process is disrupted, necrosis of the deeper cartilage cells is imminent, resulting in cartilage softening and the formation of erosions. These fragments of the medial coronoid process usually undergo calcification because their perfusion, which occurs through fibrous attachments to the annular ligament, is disrupted.
This theory contrasts with a more recent paper, which was based on the histopathologic examination of the coronoid process in clinical cases.19 According to the latter, osteochondritis dissecans lesions were not discovered in the affected segments of the medial coronoid process; however, changes consistent with fractures were detected. It is concluded that such lesions are caused by powerful and constant forces on the cartilagenous medial coronoid process and the formation of stress fractures, which inhibit the ossification of its fibrous tissue.
Β. Elbow incongruity
The elbow joint is a composite joint, formed by three specific articulations, including the humeroradial joint (the capitulum of the humeral condyle articulates with the proximal surface of the head of the radius), the humeroulnar joint (the trochlea humeri articulates with the trochlear or semilunar ulnar notch and the medial coronoid process) and the radioulnar joint (the posterior articular surface of the head of the radius articulates with the radial notch of the ulna).19
A difference in length between the radius and the ulna and humeroulnar incongruity have been previously reported as forms of incongruity.
Β1. Difference in length between the radius and the ulna (radioulnar incongruity)
This difference is caused by asynchronous longitudinal growth of the two bones. Two forms have been reported: in the first form, the radius is shorter than the ulna or the radial head is located laterally to the medial coronoid process (short radius syndrome).15,20 In the second form, the ulna is shorter than the radius or the radial physis is located medially to the medial coronoid process (short ulna syndrome).21
In the shortened radius syndrome, the magnitude of weight-bearing forces are transported by the trochlea humeri to the medial coronoid process. The intense shearing forces lead to stress-induced damage in the subchondral bone of the medial coronoid process resulting in the formation of fissures or fragmentations (Figure 1).21
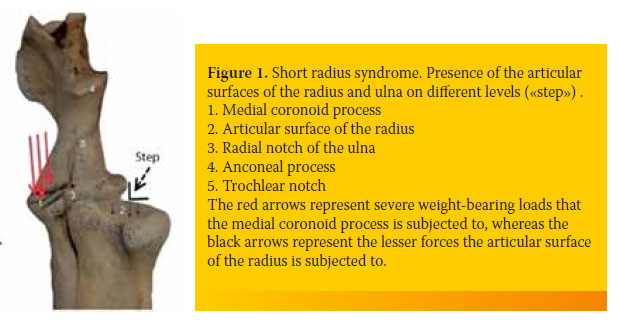
In the second form, the head of the radius exerts centrally-located pressure on the humeral condyle, which in turn is transmitted to the anconeal process, thus preventing its ossification and fusion with the ulnar physis.22,23
According to the above and based on linear forces being exerted on the joint, MCD and UAP can be explained. With the linear forces theory, however, the combination of MCD and UAP in clinical cases cannot be justified.5
In order to interpret the combination of these abnormal conditions, a new theory has been suggested based on the action of rotational forces (rotational force theory).24 According to this theory, there are once more two variations related to the difference of length between the radius and the ulna. In the shortened radius syndrome, which is less frequently observed, the linear force theory and the rotational force theory are relatively similar. In contrast, in the shortened ulna syndrome, which is more commonly reported, the rotational theory differs from the linear force theory.
According to the rotational force theory, if the radius grows in length faster than the ulna, the radial head exerts forces with a central direction to the humeral condyle. Initially, the displacement of the humerus medially is prevented by the anconeal process. Later, as the radius continues to grow in length, so do the loads placed on the humeral condyle, which begins to externally rotate. As the humerus rotates externally, the caudal aspect of the humeral condyle impinges on the lateral surface of the base of the anconeal process. If this process occurs prior to the ossification of the growth plate of the anconeal process, UAP may develop. The continuous medial advancement of the humeral bone, combined with its simultaneous rotation, places intense loads and leads to abrasions upon the point of contact of the caudal medial surface of the humeral condyle with the lateral surface of the ulnar trochlear notch. At this point, lesions caused by friction are formed in the cartilage of the humeral condyle, as well as in the cartilage of the trochlear notch. The continuous loads placed by the radial head on the humeral condyle, combined with the effect of the anconeal process as a lever, lead to the downward displacement of the trochlea humeri and the compression of the medial coronoid process. The compression and friction result in the formation of lesions in the medial coronoid process, as well as the medial part of the humeral condyle.
Finally, as rotation of the humerus continues to increase, especially when there is no UAP, rostral displacement of the trochlea humeri follows, resulting in its displacement from the trochlear notch of the ulna, thus causing elbow subluxation.
Β2. Humeroulnar incongruity
This is noted when the radius of curvature of the trochlear notch of the ulna is smaller than the radius of curvature of the trochlea humeri or when the trochlear notch of the ulna has an elliptical shape. This geometrical abnormality subsequently results in malarticulation of the trochlear humeri with the trochlear notch, resulting in abnormal forces and loads on the medial coronoid process.25,26 It has been noted that in Bernese mountain dogs there is a predilection for the formation of an elliptical trochlear notch.2
C. Posterolateral rotatory instability of the proximal radioulnar joint
When there is incongruity of the caudal articular surface of the radius with the radial notch of the ulna, the lateral pull of the biceps brachii and the brachialis muscle causes external rotation of the ulna (over the radius) during flexion of the joint. Rotation results in compression of the two articular surfaces and damage to the medial coronoid process and radial notch.27
> Diagnosis
Each form of elbow dysplasia may develop as a single condition or, more commonly, in combination. Unrelated to the form of dysplasia, clinical signs are always similar, thus inhibiting identification of the form by physical examination alone. Clinical signs usually begin from the age of 3-10 months.
During observation of dogs with elbow dysplasia, a stiff gait is noted following a period of rest, or lameness manifests after exercise. When the disorder affects both forelimbs, detection of lameness can be challenging. In a standing position, the affected limb is abducted and the antebrachium and toes are externally rotated (supination). This position helps to reduce the forces applied to the medial compartment of the elbow.28
One of the most reliable findings during physical examination is pain manifesting upon palpation as well as during passive range motions of the elbow joint. Pain manifests upon deep palpation of the insertion of the biceps brachii muscle into the ulnar tuberosity which is located on its internal surface, over the medial aspect of the coronoid process29. Moreover, pain manifests during extreme flexion of the joint in combination with external rotation of the antebrachium.
The manifestation of pain during physical examination, combined with the absence of any other recognizable cause of lameness or pain, is an important finding that supports elbow dysplasia. In the case of UAP, a synovial fluid effusion may be observed.30 In MCD, the presence of joint effusion is uncommon.
In dogs with the chronic form of the disorder and simultaneous presence of secondary osteoarthritis, a decrease in joint motion range is observed along with crepitation, muscular atrophy and swelling of the elbow joint.
Cases of adult dogs have been reported to show unilateral or bilateral lameness of the forelimbs due to MCD, with no history of lameness at a younger age.7
The differential diagnosis should always include every possible cause of frontal limb lameness such as disorders of the shoulder joint, tendon injury, neurological causes of lameness and neoplasia.
For a definitive diagnosis and identification of the form of elbow dysplasia, imaging investigation of the elbow joint is essential. Imaging can be performed with standard radiography, computed tomography (CT) or magnetic resonance imaging (MRI). Primary findings of elbow dysplasia may be missed in radiographs. In such cases, diagnosis is based on detecting secondary findings of osteoarthritis, which develop after the age of seven months.
Radiographs contribute poorly to the diagnosis of MCD. For example, erosions in the articular cartilage of the condyle or fissures in the subchondral bone of the medial coronoid process cannot be viewed with standard radiography.31,32,33,34
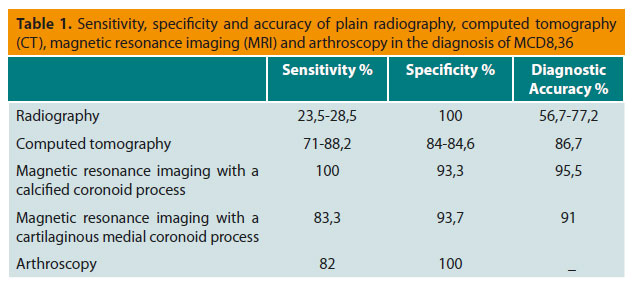
In a dog with clinical signs of elbow dysplasia, if no lesions are found on standard radiographs or if such lesions are unclear, further diagnostic investigation is warranted by CT or MRI. With CT, the bone structures of the elbow joint are viewed without superimposition.35 For this reason, its specificity and sensitivity are greater than those of radiography (Table 1).8,36
Nevertheless, it is possible that CT and MRI may not clearly visualize subchondral bone lesions in the medial coronoid process. If the findings of all imaging modalities are unclear, arthroscopy is recommended. In 37-50% of cases, the disorder affects both forelimbs, rendering imaging investigation of both joints necessary.8
For conventional radiographic evaluation, good quality radiographs are required with the dog correctly placed. It is necessary to sedate or fully anaesthetize the animals. In all positions, the limb is placed on top of the cassette without the use of a grid. Furthermore, radiography should include the shoulder joint for possible osteochondritis dissecans which may be the cause of lameness or may coexist with elbow dysplasia.
In order to depict all of the basic anatomical segments of the joint and all the possible pathological conditions, radiographs should be obtained with the limb in four separate views. The latter include the mediolateral projection with the elbow extended, the maximally flexed mediolateral projection, the craniocaudal view and external oblique or craniolateral-caudomedial oblique projection (150). In the former two views, the dog is positioned in lateral recumbency and in the latter in sternal recumbency.
A. Mediolateral projection with the elbow extended
In this view, the angle between the humerus and the antebrachium should be about 110ο. Τhe limb is positioned in such a way that there is superimposition of the humeral condyles.
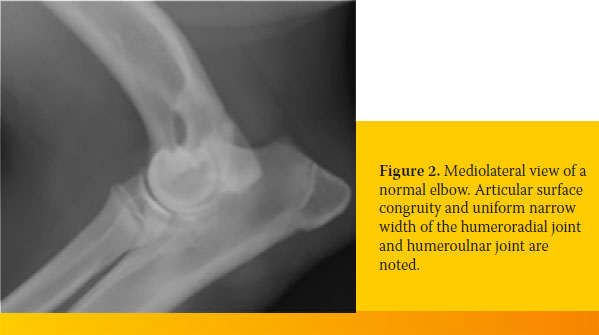
Visualization of a normal elbow in a dog older than six months is characterized by articular surface congruity and uniform narrow joint spaces between the humerus, the radius and the ulna. Additionally, the edge of the trochlear notch of the ulna and the radial head should form an arch with a smooth outline (Figure 2).
With such positioning, in a dog less than one year of age with elbow dysplasia, one or more of the following abnormal findings may be noted:
- Increased joint space in the humeroulnar articulation along the internal surface of the trochlear notch.
- Increased joint space in the humeroradial articulation
- Interruption of the smooth outline of the arch formed by the ulnar trochlear notch and the articular surface of the radius. This abnormal finding is called «step».34
- Fragmented medial coronoid process, which can be simple or compound. The normal medial coronoid process is seen as a triangular area of subchondral bone with a sharp outline, superimposing over the radial head. Fracture of the medial coronoid process is seen only in 9.8 % of cases.
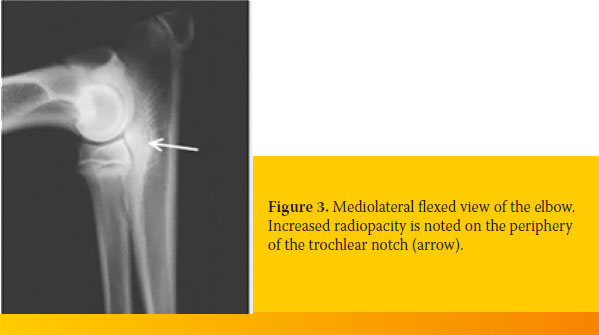
- Mild increase of bone radiopacity (osteosclerosis) and loss of trabecular bone along the medial surface of the semilunar notch of the ulna. This radiologic finding is an early indication of abnormal loads placed on the elbow joint (Figure 3).
- Presence of osteophytes along the dorsal aspect of the anconeal process. In dogs older than one year of age, lesions of secondary osteoarthritis usually extend to the proximal articular surface of the radial head.
B. Maximally flexed mediolateral projection
In this positioning, the angle between the humerus and the antebrachium should be more or less equal to 45ο. The limb is placed so that there is superimposition of the humeral condyles on the radiographic film.
This positioning is ideal for the diagnosis of UAP, compared to the mediolateral view of the extended elbow, because it has the distinct advantage of being able to avoid superimposition of the medial epicondyle on the olecranon. Therefore, the anconeal process and any osteophytes along its dorsal aspect can be clearly visualized.
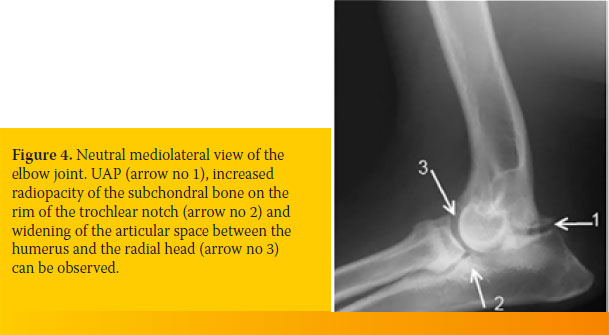
In large dog breeds, the anconeal process has its own separate secondary centre of ossification. The physis of this ossification centre of the anconeal process can be visualized in radiographs up until the age of 3½ - 5½ months. If the physis remains visible in radiographs of older dogs, it is considered pathognomonic for UAP. The remaining physis is seen as a radiolucent space with unclear margins between the anconeal process and the olecranon (Figure 4).
C. Craniocaudal view of the elbow joint
In this view, the dog is placed in sternal recumbency and the limb intended for radiographic evaluation is held with the humerus vertical to the axis of the spinal column, the elbow joint is held in contact with the cassette, and the antebrachium is rostrally extended.
In such a position, the characteristic OCD lesion can be visualized as early as 5-6 months of age as a radiolucent area or collapse of the medial compartment, or even as a defect in the subchondral bone of the articular surface of the humeral trochlea.33
D. Craniolateral-caudomedial oblique projection (-150) of the elbow.
In this view, the dog is placed in sternal recumbency and the limb intended for radiographic evaluation is positioned as for the craniocaudal view except that the antebrachium is rotated externally by 150 (pronation). The radiographic beam is focused on the centre of the elbow joint. In this position, the articular surface of the trochlea humeri and the medial coronoid process are more clearly visualized.
> Current treatment options
Treatment for elbow dysplasia is surgical and should be performed before the development of osteoarthritic lesions in the joint. If surgical treatment is delayed, the development of osteoarthritis cannot usually be adequately controlled. Unfortunately, the complexity of aetiopathogenesis, physical examination and imaging findings render diagnosis in the early stages of the disease somewhat of a challenge.
Surgical techniques to correct elbow dysplasia described in this paper are classified as surgical procedures to correct MCoD in dogs with mild osteoarthritis or moderate or severe osteoarthritis, and to correct the UAP.44
1. Surgical procedures for MCoD in dogs with mild osteoarthritis.
a.MCD.
Even though several research studies have been undertaken to select the appropriate modality in order to correct MCD, controversy still surrounds the procedure of choice up to the present day. The results of research studies vary. However, normal function of the limb has been reported in most procedures for a short length of time after surgery, and exacerbation of osteoarthritis has been noted in the mid- to long-term outcome.37,38 In fact, some authors report that surgical treatment does not alter the long-term prognosis, and they prefer to manage MCD with conservative treatment.39
Surgical procedures that have been described in the treatment of MCD can be classified under two categories. Focal procedures via arthrotomy or arthroscopy can be placed under the first category, whereas the second category includes proximal ulnar osteotomy which can be performed in combination with focal procedures.
Among the focal techniques, those that have been researched the most are the removal of the osteochondral fragment or fragments of the medial coronoid process, as well as subtotal medial coronoid ostectomy.
Removal of the fragment or fragments of the medial coronoid process always leads to osteoarthritis and persistence of lameness because degenerative lesions remain in the subchondral bone. For that reason, simultaneous partial osteotomy of a large segment of the medial coronoid process was recommended, in which most of the diseased subchondral bone is included 28,40,41 (Figure 5).
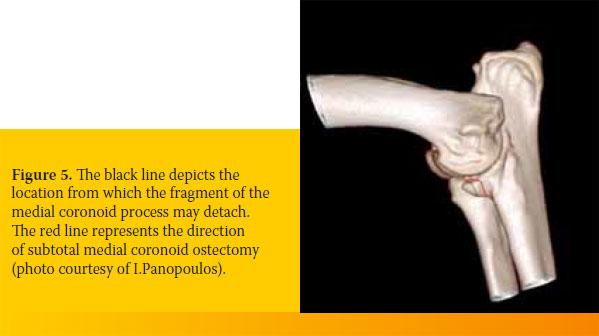
Subtotal medial coronoid ostectomy, which can be performed with an osteotome or an air saw, has a caudal medial direction from the periphery of the medial coronoid process toward the external aspect of the radial notch of the ulna.
The attachments of the annular ligament are released from the osteotomized bone fragment and the fragment itself is removed.42,43 The joint is then irrigated with normal saline and subsequently injected with 1 mg/kg body weight of a 0.5% bupivacaine solution; the surgical site is sutured.
Insufficient comparative studies have been conducted concerning the results of partial ostectomy of the medial coronoid process and the simple removal of medial coronoid process fragments. As a consequence, more research is required in order to determine the indications and postoperative outcome of the aforementioned procedures in everyday clinical practice.43
If MCD coexists with short radius syndrome, in order to manage the severe load-bearing forces placed upon the medial compartment of the elbow joint, focal treatment is combined with proximal ulnar osteotomy.44
It is common for MCD to coexist with lesions on the articular cartilage of the humeral trochlea, treatment for which includes flap removal and debridement of the area.
b.Short radius syndrome
Treatment of the short radius syndrome aims to repair the congruity between the articular surfaces of the radioulnar articulation by bringing the articular surfaces of the radius and ulna on the same level. This objective is accomplished surgically by proximal ulnar osteotomy.25,45
After ulnar osteotomy, the articular surface of the ulna is transported laterally by weight-bearing forces, resulting in alignment of the articular surfaces of the radius and ulna on the same level. This procedure is selected when in the mediolateral radiograph of the elbow joint the difference of level (step) between the articular surfaces of the radius and ulna is wider than 2 mm.
Ulnar osteotomy is performed by two parallel oblique ulnar osteotomies with a distance between them that is equal to the difference in level between the ulnar-radial articular surfaces that is visualized on the mediolateral radiograph. The osteotomies are oblique to the longitudinal axis of the ulna and their direction is caudal medial to rostrolateral with a 40o angle to the longitudinal axis and mediolateral with a 500 angle to the longitudinal axis (Figure 6).20
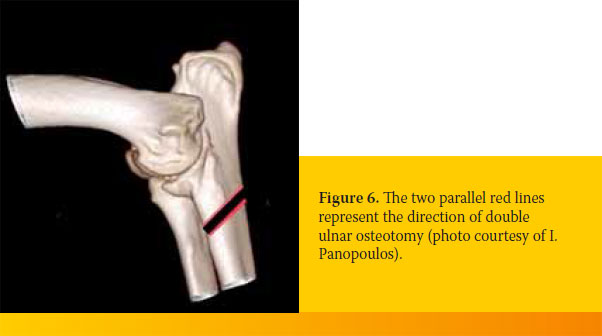
Alignment of the osteotomized ulnar fragments may be secured with an intramedullary pin.46
c.Rotatory instability of the radioulnar joint.
In order to neutralize the shearing forces formed between the radius and the ulna during flexion of the elbow joint due to existing incongruity between the caudal articular surface of the radius and the radial notch of the ulna, combined with the lateral position of the insertions of the biceps brachii and brachialis muscles to the anatomical axis of the antebrachium, a surgical technique was recently published that released the insertion tendons of the biceps brachii and brachialis muscles from the ulna (biceps-brachial ulnar release procedure, BURP).27
The insertion tendon of the biceps brachii muscle has two branches. The one branch is inserted into the radial tuberosity (medial part of the frontal aspect of the radial head), whereas the other branch, along with the tendon of the brachialis muscle is inserted into the ulnar tuberosity in the form of a wide «fan» (intra-articular medial surface of the coronoid process). In the biceps-brachial ulnar release procedure, surgical release of the insertions is performed on the inner surface of the medial coronoid process. Topical surgical access can be provided via minimally invasive arthrotomy or arthroscopy.
The BURP technique is indicated in young dogs with lameness, manifestation of pain during joint palpation, in which either imaging or arthroscopy confirm that the degree of articular cartilage and subchondral bone damage is mild (few fissures present and mild subchondral bone sclerosis of the radial notch area).28
Elbow joint flexion remains unaffected after the procedure because the remaining second insertion of the biceps brachii on the radius remains intact.
d.OCD.
The standard procedure for managing OCD of the humeral trochlea includes the removal of the detached cartilage as well as trimming part of the articular cartilage from the rim of the lesion, aiming at the removal of the inflammatory nidus from the joint. Subsequently, the rim of the defect site is debrided to create a vertical defect perimeter so that implanting fibrocartilage into the defect will not be impeded .47 Prior to suturing the articular capsule, the joint is irrigated thoroughly with normal saline to remove friction-made debris due to the cartilage flap.
In order to stimulate and promote the healing response of the articular cartilage after the removal of any detached cartilage segments, arthroplasty and cartilage restoration procedures have been described.
The goal of arthroplasty procedures is to create vascular channels from the subchondral bone to the defect area. The introduction of blood in the defect provides haematopoietic and mesenchymal stem cells as well as developmental factors to promote the healing process and improve the quality of fibrocartilage tissue formation so as to resemble hyaline cartilage more closely.
The arthroplasty techniques include curettage and osteoparacentesis.
Curettage is the removal of detached or abnormal cartilage with a curette, as well as debridement of the affected subchondral bone to the level of the healthy bleeding bone. The main disadvantage of curettage is the extensive damage to the subchondral bone, as well as the formation of a wide and deep defect, resulting in delay of healing and the creation of poor quality fibrocartilage tissue.
Osteoparacentesis involves the formation of several canals in the subchondral bone with a narrow Kirschner pin, to the point of bleeding. Compared to the curettage technique, less subchondral bone damage is incurred.
The articular cartilage replacement procedures aim at repairing the defect with hyalinous cartilage. This is accomplished by placing an osteochondral autograft in the defect site. Specifically, after removal of the osteochondral flap, an appropriate recipient socket is formed in the chondral defect to accept the osteochondral graft, which will cover the eroded area (Osteochondral Autograft Transfer System, OATS, Arthex, Naples, FL). The osteochondral graft is collected by a special process from the articular surface of the knee joint.1
2. Surgical procedures to manage MCoD in dogs with moderate or severe osteoarthritis.
Modern procedures, applied in cases of osteoarthritis lesions in the medial compartment only, include sliding humeral osteotomy (SHO) and proximal ulnar osteotomy followed by fixation of bone fragments in a new position resulting in limb abduction (proximal abducting ulnar osteotomy, PAUL). The aim of such procedures is to reduce load-bearing forces on the eroded medial compartment and transfer such forces to the healthy lateral compartment.
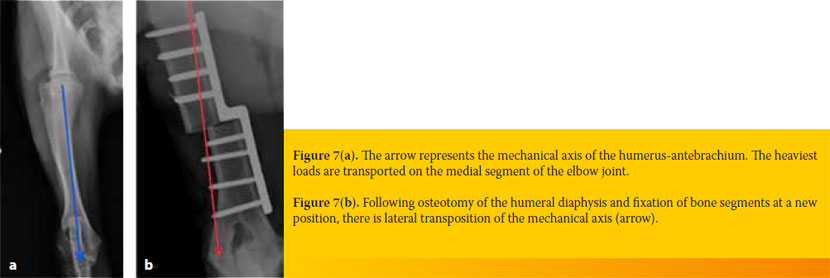
In the SHO procedure, a transverse osteotomy is performed on the middle of the humeral diaphysis and the two resulting bone fragments are fixed in a new position with a sliding metal plate, which has a «step» at its middle. With the aid of the aforementioned special sliding metal plate, the peripheral section of the humerus is translocated medially to the mechanical axis of the humerus- antebrachium. This lateral transposition of the mechanical axis results in shifting load-bearing forces away from the affected articular cartilage of the humeroulnar joint and towards the normal articular cartilage of the humeroradial joint (Figures 7a and 7b).48,49
In a study including 59 elbow dysplasia cases on which the SHO procedure was performed, a good to excellent mid-term outcome was noted.49 Specifically, no lameness was reported at 26 weeks post surgery in 65.6% of cases and in 31.3% of cases, the presence of lameness was mild. However, severe complications have been noted due to fixation of the metal plate on the humerus and it is estimated that more studies are required with a larger number of cases in order to evaluate the long-term postoperative, before this procedure can be established in the clinical setting.
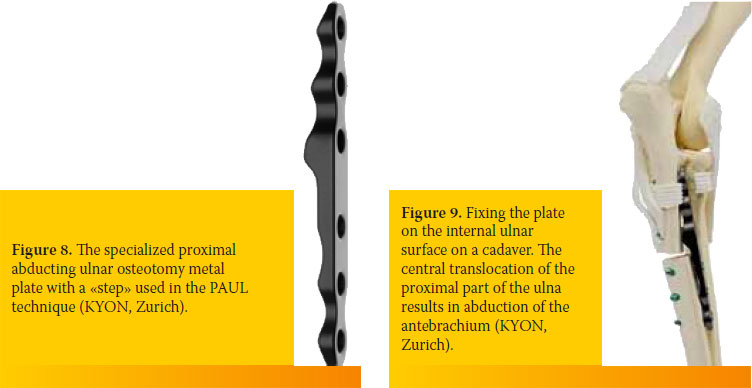
In the most recent PAUL procedure, a transverse proximal ulnar osteotomy is performed and the two ulnar fragments are fixed in a new position with a special sliding metal plate with a 2 to 3 mm «step» at its middle (KYON, Zurich, Switzerland) (Figure 8).50 In this new position, the ulna causes abduction of the antebrachium, enabling the unloading of the medial compartment and the transfer of load-bearing forces to the lateral compartment of the elbow joint (Figure 9). Even though this is a promising procedure, there have been no extensive studies to analyze the success ratio and potential complications from its use.
In cases of severe osteoarthritis lesions in both compartments of the elbow joint, surgical options include arthrodesis or total elbow replacement.
Elbow arthrodesis offers relief from pain due to chronic osteoarthritis; however, there is severe loss of function in the limb.49
Even though total elbow arthroplasty has been studied for over 10 years, it is rarely used in a clinical setting due to several technical difficulties, a high complication rate and an unpredictable outcome.51 Total elbow replacement should only be attempted in elbows with severe osteoarthritis, and only after the owner’s informed consent because there is a high risk of several complications.
3. Surgical procedures for UAP
It is possible to surgically correct UAP with three separate procedures, including proximal abduction ulnar osteotomy, removal of the anconeal process and lag screw fixation of the anconeal process on the ulna.52
Performing a proximal ulnar osteotomy results in relocating the proximal ulnar fragment medially due to the pull of the triceps brachii muscle, repairing radio-ulnar congruity as a consequence, and offering relief for the anconeal process from pressure exerted by the humeral condyle.23,53 The direction of ulnar osteotomy is similar to the direction of proximal ulnar ostectomy. The two ulnar fragments are then released or fixed with an intramedullary pin.
The procedure for lag screw fixation of the anconeal process is used on dogs younger than six months of age, with a normal trochlear notch.
The best postoperative results reported in the literature relate to screw fixation of the anconeal process combined with proximal ulnar osteotomy.54
> Conclusions
Currently, it is maintained that the main cause for elbow dysplasia is IC, even though in some dogs OCD is involved. The most common form of incongruity is the asynchronous growth of the radius and ulna, in which the radial head is at a lower or higher level than the medial coronoid process, thus re-distributing intense forces on the anconeal process or the medial coronoid process respectively. It is maintained that the exact aetiopathogenic mechanism is complex and has not yet been fully elucidated.
The definitive diagnosis requires imaging or even arthroscopy. Computed tomography has a higher specificity and sensitivity in the diagnosis of elbow dysplasia than standard radiography.
Surgical treatment for elbow dysplasia should be considered before the development of severe osteoarthritis lesions. Surgical procedures include partial resection of the medial coronoid process and repair of length difference and congruity between the radial and ulnar articular surfaces by ostectomy or osteotomy. If joint lesions are severe and affect only the medial compartment, the SHO or PAUL techniques are used. If there are lesions across the entire elbow joint, total elbow replacement or arthrodesis is recommended. Arthrodesis is a solution for pain but severe functional issues remain.
A complete understanding of the pathogenesis of elbow dysplasia will become possible after research, analysis and processing of data collected from force plate analysis. Only then will it be possible for all surgical options to be reconsidered, possibly giving rise to new modalities for the treatment of this common disease that causes chronic pain and is severely debilitating for affected dogs.
> Acknowledgements
The authors would like to thank their colleague Ioannis Panopoulos for offering photos from his personal records.
> References
1. Griffon DJ. Surgical diseases of the elbow. In: Small Animal Veterinary Surgery. Tobias KM, Johnson SA (eds). Elsevier Saunders, St Louis, MO, USA, 2012, pp.724-759.
2. Hazewinkel HAW, Meij BP, Nap RC, Ubbink GJ. Radiographic views for elbow dysplasia screening in Bernese Mountain dogs. Proceedings International Elbow Working Group 1995, 5: 32-37.
3. Morgan JP, Wind A, Davidson AP. Bone dysplasias in the labrador retriever: a radiographic study. J Am Anim Hosp Assoc 1999, 35: 332–340.
4. Morgan JP, Wind A, Davidson AP. Elbow Dysplasia. In: Hereditary Bone and Joint Diseases in the Dog. Morgan JP and Davidson AP, (eds). 1st ed. Hannover Schultersche: Germany, 2000, pp. 41-68.
5. Meyer-Lindberg A, Fehr M, Nolte I. Co-existance of UAP and FCP of the ulna in the dog. J Small Anim Pract 2006, 47: 61–65.
6. Kirberger RM, Fourie SL. Elbow dysplasia in the dog: pathophysiology, diagnosis and control. J S Afr Vet Assoc 1998, 69: 43–54.
7. Vermote KA, Bergenhuyzen AL, Gielen I, van Bree H, Duchateau L, Van Ryssen B. Elbow lameness in dogs of six years and older. arthroscopic and imaging findings of medial coronoid disease in 51 dogs. Vet Comp Orthop Traumatol 2010, 23: 43–50.
8. Snaps FR, Balligand MH, Saunders JH, Park RD, Dondelinger RF. Comparison of radiography, magnetic resonance imaging and surgical findings in dogs with elbow dysplasia. Am J Vet Res 1997, 58: 1367-1370.
9. Michelsen J. Canine Elbow dysplasia: aetiopathogenesis and current treatment recommendations. Vet J 2013, 196:12- 19.
10. Clements DN. Gene expression in normal and diseased elbows. In: Proceedings of the Autumn Meeting of the British Veterinary Orthopaedic Association: Chester, UK, 2006, pp. 6–7.
11. Grandalen J, Lingaas F. Arthrosis in the elbow joint of young rapidly growing dogs: A genetic investigation. J Small Anim Pract 1991, 32: 460–464.
12. Lewis TW, Ilska JJ, Blott SC, Woolliams JA. Genetic evaluation of elbow scores and the relationship with hip scores in UK Labrador retrievers. Vet J 2011, 189: 227–233.
13. Nap RC. Pathophysiology and clinical aspects of canine elbow dysplasia. In: Proceedings of the 7th International Elbow Working Group Meeting, Constance, Germany, 1995, pp. 6–8.
14. Olsson SE. The early diagnosis of fragmented coronoid process and osteochondritis dissecans of the canine elbow joint. J Am Anim Hosp Assoc 1983, 19: 616–626.
15. Gemmill TJ, Mellor DJ, Clements DN, Clarke SP, Farrell M, Bennett D, Carmichael S. Evaluation of elbow incongruency using reconstructed CT in dogs suffering fragmented coronoid process. J Small Anim Pract 2005, 46: 327–333.
16. Kramer A, Holsworthy IG, Wisner ER, Kass PH, Schultz KS. Computed tomographic evaluation of canine radioulnar incongruence in vivo. Vet Surg 2006, 35: 24–29.
17. Hulse D. Co-contraction of the biceps/brachialis muscle complex produces a rotational moment which may induce fragmentation/microfracture of the medial coronoid. In: Congress proceedings of the American College of Veterinary Surgeons Symposium: San Diego, USA, 2008, pp. 466.
18. Bennett D, Duff SR, Kene RO, Lee R. Osteochondritis dissecans and fragmentation of the coronoid process in the elbow joint of the dog. Vet Rec 1981, 109: 329–336.
19. Guthrie S, Plummer JM, Vaughan LC. Post natal development of the canine elbow joint: a light and electron microscopic study. Res Vet Sci 1992, 52: 67-71. 20. Burton, NJ, Owen MR. Canine elbow dysplasia 2. Treatment and prognosis. In Practice 2008, 30: 552–557.
21. Bottcher P. Radio-ulnar incongruence in dogs with elbow dysplasia. In: Congress proceedings of the American College of Veterinary Surgeons Symposium, Chicago, USA, 2011b, pp. 110–112.
22. Preston, CA, Schulz KS, Kass PH. In vitro determination for contact areas in the normal elbow joint of dogs. Am J Vet Res 2000, 61: 1315–1321.
23. Sjstrom L, Kasstom H, Kllber M. Ununited anconeal process in the dog. Pathogenesis and treatment by osteotomy of the ulna. Vet Comp Orthop Traumatol 1995, 8: 170–176.
24. Lozier SM. How I treat elbows in the older canine patient and new prospective in elbow dysplasia. In: Congress proceedings of the 13th European Society of Veterinary Orthopaedics and Traumatology: Munich, Germany, 2006, pp. 93–96.
25. Wind AP, Packard ME. Elbow incongruity and developmental elbow diseases in the dog. Part II. J Am Anim Hosp Assoc 1986, 22: 725–730.
26. Proks P, Necas A, Stehlik L, Srnec R, Griffon DJ. Quantification of humeroulnar incongruity in Labrador retrievers with and without medial coronoid disease. Vet Surg 2011, 40: 981–986.
27. Fitzpatrick N. Biceps ulnar release procedure for treatment of medial coronoid disease in 49 elbows. In: Congress proceedings of the 36th Annual Conference, Veterinary Orthopaedic Society, Steamboat Springs: Colorado, USA, 2009, p. 44.
28. Fitzpatrick, N, Yeadon R. Working algorithm for treatment decision making for developmental disease of the medial compartment of the elbow in dogs. Vet Surg 2009, 38: 285–300.
29. Trostel C, McLaughlin R, Pool R. Canine lameness caused by developmental orthopedic diseases: FCP and UAP. Compend Cont Educ Pract 2003, 25: 112.
30. Demko J, McLaughlin R. Developmental orthopedic disease. Vet Clin North Am 2005, 35: 1111-1135.
31. Punke JP, Hulse DA, Kerwin SC, Peycke LE, Budsberg SC. Arthroscopic documentation of elbow cartilage pathology in dogs with clinical lameness without changes on standard radiographic projections. Vet Surg 2009, 38: 209
32. Boulay JP. Fragmented medial coronoid process of the ulna in the dog. Vet Clin North Am 1998, 28: 449-458.
33. Henry WB. Radiographic diagnosis and surgical management of fragmented medial coronoid process in dogs. J Am Vet Med Assoc 1984, 184: 799-805.
34. Wosar M, Lewis D, Neuwirth L, Parker RB, Spencer CP, Kubilis PS, Stubbs WP, Murphy ST, Shiroma JT, Stallings JT, Bertrand SG. Radiographic evaluation of elbow joints before and after surgery in dogs with possible fragmented medial coronoid process. J Am Vet Med Assoc 1999, 214: 52-58.
35. Rovesti G, Biasibetti M, Schumacher A, Fabiani M. The use of CT in the diagnostic protocol of the elbow in the dog: 24 joints. Vet Comp Orthop Traumatol 2002, 15: 35-39.
36. Carpenter LG, Schwarz PD, Lowry JE, Steyn PF. Comparison of radiologic imaging techniques for diagnosis of FCP of the cubital joint in dogs. J Am Vet Med Assoc 1993, 203: 78-83.
37. Ness M. Treatment of FCP in young dogs by proximal ulnar osteotomy. J Small Anim Pract 1998, 39:15-21.
38. Read RA, Armstrong SJ, O’Keef D, Eger CE. Fragmentation of the medial coronoid process of the ulna in dogs: a study of 109 cases. J Small Anim Pract 1990, 31: 330-334.
39. Book GR, Miller CW, Tavers CL. A comparison of surgical and medical treatment of fragmented coronoid process and osteochondritis dissecans of the canine elbow. Vet Comp Orthop Traumatol 1995, 8: 117-120.
40. Fitzpatrick N. Subtotal coronoid ostectomy (SCO) for the treatment of medial coronoid disease: A prospective study of 228 dogs (389 elbows) evaluating short and medium term outcome. In: Congress proceedings of the British Veterinary Orthopaedic Association, Autumn Scientific Meeting- Enigmas of the Canine Elbow, Chester, UK, 2006, pp. 22–29.
41. Danielson KC, Fitzpatrick N, Muir P, Manley PA. Histomorphometry of fragmented medial coronoid process in dogs: a comparison of affected and normal coronoid processes. Vet Surg 2006, 35: 501-512.
42. Tobias T, Miyabayashi T, Olmstead ML. Surgical removal of FCP in the dog: comparative effects of surgical approach and age at time of surgery. J Am Anim Hosp Assoc 1994, 30: 360-362.
43. Bοttcher, P. Accelerated cartilage loss following subtotal coronoid ostectomy. In: Congress proceedings of the American College of Veterinary Surgeons Symposium: Chicago, USA, 2011, pp. 108–109.
44. Preston CA, Schulz KS, Taylor KT, Kass PH, Hagan CE, Stover SM. In vitro experimantal study of the effect of radial shortening and ulnar ostectomy on contact patterns in the elbow joint of dogs. Am J Vet Res 2001, 62: 1548–1556.
45. Holsworth IG. How I manage elbow incongruity. In: Congress proceedings of the 12th European Society of Veterinary Orthopaedics and Traumatology Congress: Munich, Germany, 2004, pp. 78-79.
46. Fox DJ. Radius and ulna. In: Small Animal Veterinary Surgery. Tobias KM, Johnson SA (eds). Elsevier Saunders: St. Louis, MO, USA, 2012, pp. 761–784.
47. Johnston SA. Osteochondritis dissecans of the humeral head. Vet Clin North Am Small Anim Pract 1998, 28: 33-40.
48. Schulz, KS, Fitzpatrick N, Young R. Theory and development of the sliding humeral osteotomy. In: Congress proceedings of the American College of Veterinary Surgeons Symposium, Chicago, USA, 2011, pp. 263–265.
49. Fitzpatrick N, Yeadon R, Smith TJ, Schulz K. Techniques of application and initial clinical experience with sliding humeral osteotomy for treatment of medial compartment disease of the canine elbow. Vet Surg 2009, 38: 261–278.
50. Pfeil I, Torrington A, Vezzoni A. In proceedings: Proximal abducting ulnar osteotomy. KYON Symposium, Zurich, 2014.
51. Acker R, Van Der Meulen GT. Tate elbow preliminary trials. In: Congress proceedings of the 35th Veterinary Orthopedic Society Annual Conference, MT, USA, March 2008 pp. 49-52.
52. Meyer-Lindenberg A, Fehr M, Nolte I. Short and longterm results after surgical treatment of an ununited anconeal process in the dog. Vet Comp Orthop Traumatol 2001, 14: 101–110.
53. Turner BM, Abercromby Rh, Innes J, McKee WM, Ness MG. Dynamic proximal ulnar osteotomy for the treatment of ununited anconeal process in 17 dogs. Vet Comp Orthop Traumatol 1998, 11: 76-80.
54. Krotscheck U, Hulse DA, Bahr A, Jerram RM. Ununited anconeal process: Lag-screw fixation with proximal ulnar osteotomy. Vet Comp Orthop Traumatol 2000, 13: 212-216.



