an emerging tick-borne infectious disease
> Abstract
Anaplasma phagocytophilum, an intracellular, gram-negative aerobic bacterium, is the cause for granulocytic anaplasmosis in people, horses, dogs, cats, wolves, cattle and small ruminants. A. phagocytophilum mainly targets neutrophils and rarely eosinophilic granulocytes; in Europe it is transmitted by the tick Ixodes ricinus. A significant number of small mammals and deer comprise the reservoir for the microorganism in the wild. The majority of infected dogs remain asymptomatic, whereas those that develop clinical signs mostly present with non-specific signs like fever, depression or lethargy, anorexia and lameness. The most common laboratory finding of anaplasmosis is thrombocytopenia. Diagnosis is based on finding aggregates of the organism (morulae) in the cytoplasm of neutrophils, serological detection of specific antibodies and polymerase chain reaction. The treatment of choice is Doxycycline, administered per os at a dose of 5 mg/kg Β.W./12 hours for 2-4 weeks.
> Etiology
Canine granulocytic anaplasmosis (GA) is caused by the bacterium Anaplasma phagocytophilum (order: Rickettsiales, family: Anaplasmataceae). Previously known as Ehrlichia equi, A. phagocytophila or the causative factor of granulocytic ehrlichiosis in people, this microorganism was renamed A. phagocytophilum after recent modifications to the classification of species in the families Rickettsiaceae and Anaplasmataceae, based on the nucleotide sequence of 16S rRNA and groESL genes.1-3 A. phagocytophilum includes multiple strains that differ in terms of their geographic location, the animal species they infect, and their infectious potential. For instance, strains that were isolated in Europe did not cause experimental disease in horses, and strains that were isolated from people in the U.S.A. failed to cause overt disease in cattle.4 Moreover, the rate of morbidity and mortality of human GA in Europe appears to be lower compared to that in the U.S.A. 5
A. phagocytophilum are gram-negative, aerobic and obligatory intracellular bacteria, cocci or curved rods in shape, and 0.2-2.0 μm in diameter. They mainly target neutrophils and rarely eosinophilic granulocytes. The microorganisms are located in the cytoplasm of infected cells within vacuoles formed by the cellular membrane, and they multiply by division, forming 20 or more bacteria. The latter aggregate, thus forming the typical morula with a 1.5-2.5 μm diameter.6
> Epidemiology
Up to the present day, infection caused by A. phagocytophilum has only been detected in the Northern hemisphere where ticks of the Ixodes genus (I. persulcatus complex) are the main intermediate hosts.6,7 In Europe, the main vector is I. ricinus, although the role that other species of ticks may play cannot be excluded. For example, in Sardinia, it was detected with polymerase chain reaction (PCR) in one of 50 (2%) examined ticks of the species Rhipicephalus sanguineus.8 Trans-stadial transmission of the bacteria occurs in the tick, 3,9 and natural reservoirs of the former include a variety of species, mostly wild rodents and ruminants (deer). 1
A. phagocytophilum has been identified by PCR in various species of mammals in nearly all European countries. Other than domestic ruminants, dogs, cats, horses, donkeys, European buffalo, red deer, elk, roe deer, wild boar, wolves, Eurasian lynxes, red foxes, hares, small rodents and men have also been found to be infected.10,11 However, the disease (GA) has been substantiated only in dogs, cats, horses, cattle, sheep, goats, people, 6 and recently in wolves.6,10 It is worthy of note that since dogs and people are incidental hosts and develop bacteremia of a short duration (<28 days), their role as reservoirs of the bacterium does not seem to be crucial.12 On the contrary, migratory birds may be of great epidemiological significance through the spreading of infected ticks across long distances.6, 13-15
On rare occasions, A. phagocytophilum transmission can be accomplished without the mediation of ticks.7 The above can occur through experimental inoculation or blood transfusion, and also through hospital-acquired infections as well as infection of people from deer carcasses during the skinning process.7,16 Transmission through the placenta has been proven in cattle in which A. phagocytophilum was also found in the white blood cells of milk, following experimental infection.17,18 However, in a recent case of a pregnant bitch with GA, no perinatal transmission of the bacteria was noted in any of the five puppies of that litter.19
The frequency of canine infection by A. phagocytophilum has been extensively studied based on serologic testing; however, few epidemiological studies are based on molecular methods. Comparison of results between studies is challenging due to important differences in design (e.g. testing of samples from healthy or sick dogs, or from dogs admitted to primary veterinary clinics or second opinion clinics, and the season of sampling).6 Moreover, seropositivity does not exclusively reflect the exposure of dogs to A. phagocytophilum, since there may be serological cross-reactions with other Anaplasma species, such as A. platys.7 Despite the above limitations, the percentages of seropositive dogs in multiple European countries vary from 5% up to 70.5%,6 which renders canine GA an important emerging tick borne infectious disease. Furthermore, cases have been reported in Austria,20 France,21 Germany,22 Switzerland,23 the United Kingdom,24 Spain,25 Italy, 26,27 Poland,28 Portugal,29 Slovakia,30 Slovenia, 31,32 and Sweden. 33,34 In our country, GA has been diagnosed in some dogs based on cytological, serological, and molecular examinations,35,36 whereas bleeding diathesis was recently reported in a ram that was attributed to A. phagocytophilum infection based on serology results.37 Finally, Α. phagocytophilum DNA has been found in I. ricinus ticks.38
Infection of dogs by A. Phagocytophilum and the development of overt clinical manifestations of GA depend on the season, age, breed and on other co-infections.7 In the U.S.A., GA is more commonly reported from spring to the beginning of summer as well as in autumn,6 whereas in Germany 17 out of 18 dogs were diagnosed between April and September.39 The percentage of seropositive dogs increases with age;40 the mean age of dogs with GA ranges between 6 and 8 years.34,39,41,42 About half of the infected dogs in one study were Golden Retrievers, whereas in other studies no breed predilection was noted.34 Coexisting vector-borne infections can affect the clinical signs and laboratory findings of the disease.7 Coinfections by A. phagocytophilum and Borrelia burgdorferi sensu lato are common in North America 6 as well as in Europe,43-46 since both of these organisms are transmitted by the same tick. Coinfections with microorganisms of the genera Ehrlichia, Bartonella, Rickettsia and Babesia are also relatively common.7
> Pathogenesis
Ticks of the genus Ixodes need 24 to 48 hours from the moment of their attachment on the host to inoculation with A. phagocytophilum. Τhe bacteria possess complex mechanisms employed to evade neutrophil defenses. Specifically, once they attach themselves to these cells with the aid of P-selectin (CD62-P) and enter the cytoplasm by endocytosis, they modify several fundamental neutrophil functions in order to survive and multiply. As a result, the phagolysosomic mechanism and the production of hydrogen peroxide in the phagosomes are blocked, the phagocytosing ability and attachment of neutrophils to the vascular endothelium are reduced, and the apoptosis of infected neutrophils is delayed.7
The incubation period of the disease ranges from one to two weeks; the responsible pathogenetic mechanisms have not yet been fully elucidated. These may include myelosuppression from produced cytokines, the abnormalities in hematopoietic stem cell maturation in the bone marrow, the immunological destruction of blood cells, the malfunction of neutrophils and the hyperconsumption of platelets.6
> Clinical signs and laboratory findings
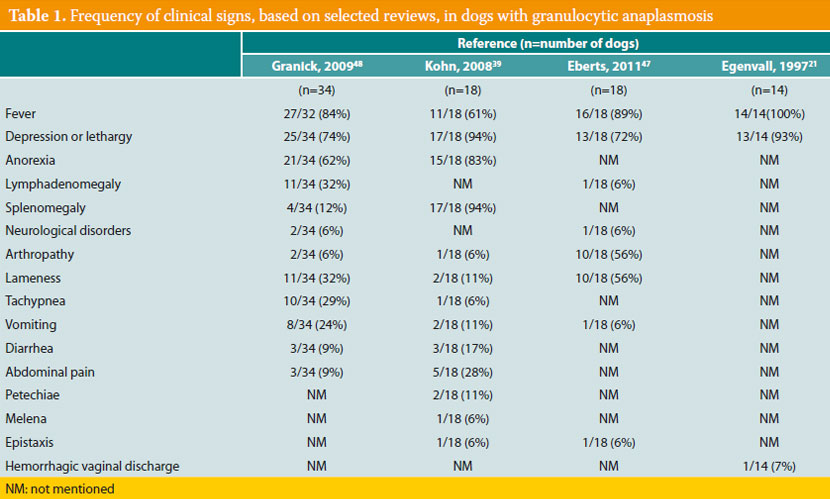
Most infected dogs remain clinically healthy;7 in the opposite case, clinical signs of the acute disease develop. Although several dogs remain subclinical carriers of the microorganism for several months, the presence of a chronic disease similar to that of canine monocytic ehrlichiosis (Ε. canis) has not been proven in the case of GA. Moreover, up until the present day, mortal cases of canine GA have not been reported.6 Τhe most typical clinical signs of canine GA (Table 1) are fever and depression or lethargy, noted in about 90% of cases;34,39,41,47,48 anorexia is also particularly common.41,42,48 Peripheral lymphadenomegaly is found in about 5-30% of cases, and splenomegaly at a percentage ranging from 10 to 100%, depending on the study.49 Lameness due to polyarthritis, unwillingness to ambulate, and musculoskeletal pain are quite frequently reported,41,50 whereas rarer clinical manifestations include vomiting, diarrhea, abdominal pain,50-52 polyuria, polydipsia, tachypnea, dyspnea, coughing, bleeding diathesis, uveitis and several neurological abnormalities such as seizures, ataxia, vestibular syndrome, and clinical signs of meningitis.
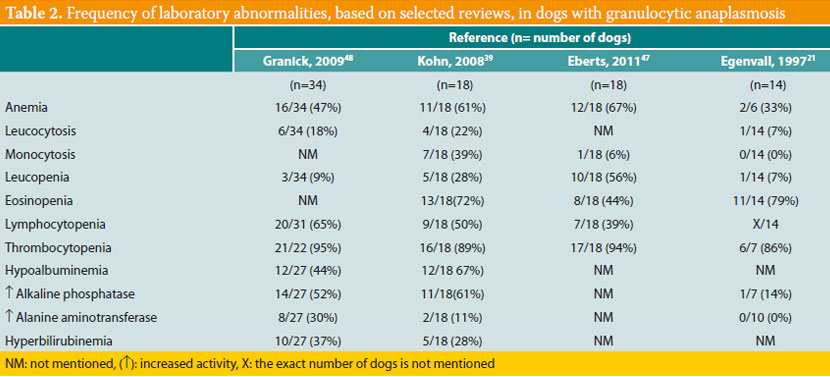
Thrombocytopenia is the most common laboratory abnormality, found in about 90% of affected dogs (Table 2). Mild non-regenerative anemia, increase or decrease in the number of white blood cells, lymphocytopenia, neutrophilia and neutropenia are noted less frequently. Hypoalbuminemia, hyperglobulinemia and a mild increase in the activity of liver enzymes (alkaline phosphatase and alanine aminotransferase) are the main serum chemistry abnormalities, whereas hyperbilirubinemia is less common.39,41,42,47,48
> Diagnosis
Other than the case history (e.g. recent exposure to ticks) and compatible clinical and laboratory manifestations, the presence of GA is confirmed when one or more of the following criteria are observed, which ultimately comprise the foundation for the diagnostic algorithm of GA in people:7 (a) the presence of morulae inside neutrophils, along with a specific antibody titer determined by an indirect immunofluorescence assay (IFA)≥1/80, (b) a fourfold increase in antibody titer between a pair of serum samples obtained with a 3-4 week interim, (c) positive PCR using specific primers for A. phagocytophilum, or (d) isolation of A. phagocytophilum from blood by culture in specific cell lines.12
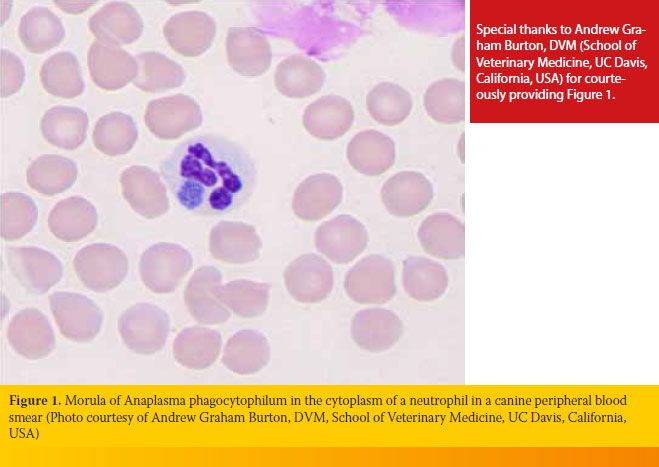
Cytological examination of peripheral blood or buffy coat smears dyed with Romanowsky-type stains (e.g. Diff-Quik, Giemsa) has a superior diagnostic sensitivity in the initial stages of GA, because morulae (Figure 1) can be detected in up to 32% of neutrophils.35 Morulae appear four days after dogs are experimentally infected and remain in high numbers for 4-8 days. Differentiating morulae of A. phagocytophilum from Ehrlichia ewingii, which can also be located in neutrophils, is impossible under the optical microscope alone; this can only be accomplished with PCR.7
In clinical practice, diagnosing GA is usually based on detecting specific antibodies in a pair of serum samples obtained with a 3-4 week interim, because a fourfold increase in titer is a strong indication of active disease. On the contrary, isolated positive antibody titers do not confirm active infection, since they may be caused by a previous infection by A. phagocytophilum and persist for a long time (up to 12 months) after its eradication (treatment or spontaneous cure). Notably, the development of symptoms in GA may precede the presence of a detectable antibody titer.7 For example, with IFA the IgG immunoglobulins can be detected in about eight days from infection and 2-5 days after the appearance of morulae in neutrophils. Nowadays, an in-clinic immunoenzymatic serologic test (ELISA) is commercially available, utilizing recombinant protein Msp2/p44 for a qualifying estimation (positive or negative result) of the presence or not of antibodies against A. phagocytophilum.53 Using this test, crossreactivity between A. phagocytophilum and A. platys has been reported, but not with E. canis.53,54
Conventional PCR and real-time PCR, combined with nucleotide sequencing, have a high diagnostic sensitivity and specificity and have been used to trace DNA of A. phagocytophilum in the blood, bone marrow and spleen.7 A negative result does not rule out infection, since the number of bacteria in the sample may be less than the diagnostic threshold, which can be expected after administration of antimicrobial drugs.16
A. phagocytophilum can be isolated by blood culture in human promyelocytic leukemia cell lines (HL-60) or in tick embryo cell lines. Despite its superior diagnostic sensitivity, this method is usually applied in research and not in the clinical setting.7
> Treatment and prognosis
The treatment of choice for canine GA is Doxycycline per os at a dose of 5 mg/kg B.W., every 12 hours for 2-4 weeks.6,7 Most dogs show clinical improvement within 24-48 hours after the initiation of treatment,7 although it has not yet been clarified whether this treatment leads to a microbiological cure. Other antimicrobials sufficient for clinical improvement are fluoroquinolones (e.g. enrofloxacin, levofloxacin) 6,16 and rifampicin, which has been previously used against the same disease in people.16
Given that there is no vaccine against A. phagocytophilum, prevention is based on avoidance of dog exposure to ticks, manual removal of the latter by special forceps, regular use of ectoparaciticides (e.g. fipronil, amitraz, pyrethroids) and the possible pre-emptive administration of doxycycline for a few days if dogs are to travel to endemic regions.6,16,22,55
> Public health significance
Human GA is an important emerging infectious disease, mainly manifesting with fever, muscular tremors, headaches and myalgia. Most patients are exposed to ticks 1-2 weeks prior to developing clinical symptoms. Advanced age and coexisting infections increase the severity of the disease. Approximately half of the patients need to be hospitalized, whereas 17% will require intensive care. Although mortality is low (0.5-1%), significant complications of the disease may occur, such as respiratory failure, opportunistic viral or fungal infections, rhabdomyolysis, acute renal failure and demyelinating polyneuropathy.56 In the U.S.A., more than 700 cases are reported each year.6 In Europe, Austria, Spain, Italy, Latvia, Norway, Netherlands, Poland, and Sweden have recorded a small number of patients;56 in Greece, 20% of healthy blood donors have been found to be seropositive to Α. phagocytophilum, whereas six cases confirmed by molecular methods were recently reported in Crete.57, 58
> References
1. Lillini E, Macri G, Proietti G, Scarpulla M. New findings on anaplasmosis caused by infection with Anaplasma phagocytophilum. Ann N Y Acad Sci 2006, 1081: 360-370.
2. Woldehiwet Z. The natural history of Anaplasma phagocytophilum. Vet Parasitol 2010, 167: 108-122.
3. Dumler JS, Barbet AF, Bekker CP, Dasch GA, Palmer GH, Ray SC, Rikihisa Y, Rurangirwa FR. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol 2001, 51: 2145-2165.
4. Pusterla N, Pusterla JB, Braun U, Lutz H. Experimental cross-infections with Ehrlichia phagocytophila and human granulocytic ehrlichia-like agent in cows and horses. Vet Rec 1999, 145: 311-314.
5. Blanco JR, Oteo JA. Human granulocytic ehrlichiosis in Europe. Clin Microbiol Infect 2002, 8: 763-772.
6. Diniz PP, Breitschwerdt EB. Anaplasma phagocytophilum infection (Canine granulocytotropic anaplasmosis). In: Infectious diseases of the dog and cat. Greene CE (ed). 4th edn. Elsevier/Saunders: St. Luis, Missouri, 2012, pp. 244-254.
7. Carrade DD, Foley JE, Borjesson DL, Sykes JE. Canine granulocytic anaplasmosis: a review. J Vet Intern Med 2009, 23: 1129-1141.
8. Alberti A, Addis MF, Sparagano O, Zobba R, Chessa B, Cubeddu T, Parpaglia ML, Ardu M Pittau M. Anaplasma phagocytophilum, Sardinia, Italy. Emerg Infect Dis 2005, 11: 1322-1324.
9. Ogden NH, Bown K, Horrocks BK, Woldehiwet Z, Bennett M. Granulocytic Ehrlichia infection in ixodid ticks and mammals in woodlands and uplands of the U.K. Med Vet Entomol 1998, 12: 423-429.
10. Leschnik M, Kirtz G, Viranyi Z, Wille-Piazzai W, Duscher G. Acute granulocytic anaplasmosis in a captive timber wolf (Canis lupus occidentalis). J Zoo Wildl Med 2012, 43: 645-648.
11. Stuen S. Anaplasma phagocytophilum - the most widespread tick-borne infection in animals in Europe. Vet Res Commun 2007, 31Suppl 1: 79-84.
12. Bakken JS, Dumler S. Human granulocytic anaplasmosis. Infect Dis Clin North Am 2008, 22: 433-448. 13. Bjoersdorff A, Bergstrom S, Massung RF, Haemig PD, Olsen B. Ehrlichia-infected ticks on migrating birds. Emerg Infect Dis 2001, 7: 877-879. 14. Palomar AM, Santibanez P, Mazuelas D, Roncero L, Santibanez S, Portillo A, Oteo JA. Role of birds in dispersal of etiologic agents of tick-borne zoonoses, Spain, 2009. Emerg Infect Dis 2012, 18: 1188-1191.
15. Hildebrandt A, Franke J, Meier F, Sachse S, Dorn W, Straube E. The potential role of migratory birds in transmission cycles of Babesia spp., Anaplasma phagocytophilum, and Rickettsia spp. Ticks Tick Borne Dis 2010, 1: 105-107.
16. Little SE. Ehrlichiosis and anaplasmosis in dogs and cats. Vet Clin North Am Small Anim Pract 2010, 40: 1121-1140.
17. Pusterla N, Braun U, Wolfensberger C, Lutz H. Intrauterine infection with Ehrlichia phagocytophila in a cow. Vet Rec 1997, 141: 101-102.
18. Pusterla N, Huder J, Wolfensberger C, Braun U, Lutz H. Laboratory findings in cows after experimental infection with Ehrlichia phagocytophila. Clin Diagn Lab Immunol 1997, 4: 643- 647.
19. Plier ML, Breitschwerdt EB, Hegarty BC, Kidd LB. Lack of evidence for perinatal transmission of canine granulocytic anaplasmosis from a bitch to her offspring. J Am Anim Hosp Assoc 2009, 45: 232-238.
20. Stadtbaumer K, Leschnik MW, Nell B. Tick-borne encephalitis virus as a possible cause of optic neuritis in a dog. Vet Ophthalmol 2004, 7: 271-277.
21. Domingos MC, Trotta M, Briend-Marchal A, Medaille C. Anaplasmosis in two dogs in France and molecular and phylogenetic characterization of Anaplasma phagocytophilum. Vet Clin Pathol 2011, 40: 215-221.
22. Kohn B, Silaghi C, Galke D, Arndt G, Pfister K. Infections with Anaplasma phagocytophilum in dogs in Germany. Res Vet Sci 2011, 91: 71-76.
23. Pusterla N, Pusterla JB, Deplazes P, Wolfensberger C, Muller W, Horauf A, Reusch C, Lutz H. Seroprevalence of Ehrlichia canis and of canine granulocy.tic Ehrlichia infection in dogs in Switzerland. J Clin Microbiol 1998, 36: 3460-3462.
24. Shaw SE, Binns SH, Birtles RJ, Day MJ, Smithson R, Kenny MJ. Molecular evidence of tick-transmitted infections in dogs and cats in the United Kingdom. Vet Rec 2005, 157: 645-648.
25. Tabar MD, Francino O, Altet L, Sanchez A, Ferrer L, Roura X. PCR survey of vectorborne pathogens in dogs living in and around Barcelona, an area endemic for leishmaniasis. Vet Rec 2009, 164: 112-116.
26. Manna L, Alberti A, Pavone LM, Scibelli A, Staiano N, Gravino AE. First molecular characterization of a granulocytic Ehrlichia strain isolated from a dog in South Italy. Vet J 2004, 167: 224-227.
27. Gravino AE, De Caprariis D, Manna L, Cerundolo R, Sagazio P, Buonavoglia C. Preliminary report of infection in dogs related to Ehrlichia equi: description of three cases. New Microbiol 1997, 20: 361-363.
28. Skotarczak B, Adamska M, Rymaszewska A, Supron M, Sawczuk M, Maciejewska A. [Anaplasma phagocytophila and protozoans of Babesia genus in dogs from endemic areas of Lyme disease in north-western Poland]. Wiad Parazytol 2004, 50: 555-561.
29. Santos AS, Alexandre N, Sousa R, Nuncio MS, Bacellar F, Dumler JS. Serological and molecular survey of Anaplasma species infection in dogs with suspected tickborne disease in Portugal. Vet Rec 2009, 164: 168-171.
30. Majlathova V, Majlath I, Vichova B, Gul’ova I, Derdakova M, Sesztakova E, Pet’ko B. Polymerase chain reaction confirmation of Babesia canis canis and Anaplasma phagocytophilum in dogs suspected of babesiosis in Slovakia. Vector Borne Zoonotic Dis 2011, 11: 1447-1451.
31. Tozon N, Petrovec M, Avsic-Zupanc T. Clinical and laboratory features of the first detected cases of A. phagocytophila infections in dogs from Slovenia. Ann N Y Acad Sci 2003, 990: 424-428.
32. Ravnik U, Tozon N, Smrdel KS, Zupanc TA. Anaplasmosis in dogs: the relation of haematological, biochemical and clinical alterations to antibody titre and PCR confirmed infection. Vet Microbiol 2011, 149: 172-176.
33. Johansson KE, Pettersson B, Uhlen M, Gunnarsson A, Malmqvist M, Olsson E. Identification of the causative agent of granulocytic ehrlichiosis in Swedish dogs and horses by direct solid phase sequencing of PCR products from the 16S rRNA gene. Res Vet Sci 1995, 58: 109-112.
34. Egenvall AE, Hedhammar AA, Bjoersdorff AI. Clinical features and serology of 14 dogs affected by granulocytic ehrlichiosis in Sweden. Vet Rec 1997, 140: 222-226.
35. Mylonakis ME, Koutinas AF, Baneth G, Polizopoulou Z, Fytianou A. Mixed Ehrlichia canis, Hepatozoon canis, and presumptive Anaplasma phagocytophilum infection in a dog. Vet Clin Pathol 2004, 33: 249-251.
36. Mylonakis ME, Koutinas AF, Breitschwerdt EB, Hegarty BC, Billinis CD, Leontides LS, Kontos VS. Chronic canine ehrlichiosis (Ehrlichia canis): a retrospective study of 19 natural cases. J Am Anim Hosp Assoc 2004, 40: 174-184.
37. Giadinis ND, Chochlakis D, Ioannou I, Kritsepi-Konstantinou M, Papadopoulos E, Psaroulaki A, Karatzias H. Haemorrhagic diathesis in a ram with Anaplasma phagocytophilum infection. J Comp Pathol 2011, 144: 82-85.
38. Kachrimanidou M, Papa A, Chochlakis D, Pavlidou V, Psaroulaki A. Molecular evidence for Anaplasma phagocytophilum in Ixodes ricinus ticks from Greece. Vector Borne Zoonotic Dis 2011, 11: 1391-1393.
39. Kohn B, Galke D, Beelitz P, Pfister K. Clinical features of canine granulocytic anaplasmosis in 18 naturally infected dogs. J Vet Intern Med 2008, 22: 1289-1295.
40. Egenvall A, Bonnett BN, Gunnarsson A, Hedhammar A, Shoukri M, Bornstein S, Artursson K. Sero-prevalence of granulocytic Ehrlichia spp. and Borrelia burgdorferi sensu lato in Swedish dogs 1991-94. Scand J Infect Dis 2000, 32: 19-25.
41. Greig B, Asanovich KM, Armstrong PJ, Dumler JS. Geographic, clinical, serologic, and molecular evidence of granulocytic ehrlichiosis, a likely zoonotic disease, in Minnesota and Wisconsin dogs. J Clin Microbiol 1996, 34: 44-48.
42. Poitout FM, Shinozaki JK, Stockwell PJ, Holland CJ, Shukla SK. Genetic variants of Anaplasma phagocytophilum infecting dogs in Western Washington State. J Clin Microbiol 2005, 43: 796-801.
43. Smith FD, Ballantyne R, Morgan ER, Wall R. Estimating Lyme disease risk using pet dogs as sentinels. Comp Immunol Microbiol Infect Dis 2012, 35: 163-167.
44. Savic S, Vidic B, Lazic S, Lako B, Potkonjak A, Lepsanovic Z. Borrelia burgdorferi in ticks and dogs in the province of Vojvodina, Serbia. Parasite 2010, 17: 357-361.
45. Pantchev N, Schaper R, Limousin S, Norden N, Weise M, Lorentzen L. Occurrence of Dirofilaria immitis and tick-borne infections caused by Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato and Ehrlichia canis in domestic dogs in France: results of a countrywide serologic survey. Parasitol Res 2009, 105 Suppl 1: S101-114.
46. Rymaszewska A, Adamska M. Molecular evidence of vector-borne pathogens coinfecting dogs from Poland. Acta Vet Hung 2011, 59: 215-223.
47. Eberts MD, Vissotto de Paiva Diniz PP, Beall MJ, Stillman BA, Chandrashekar R, Breitschwerdt EB. Typical and atypical manifestations of Anaplasma phagocytophilum infection in dogs. J Am Anim Hosp Assoc 2011, 47: 86-94.
48. Granick JL, Armstrong PJ, Bender JB. Anaplasma phagocytophilum infection in dogs: 34 cases (2000-2007). J Am Vet Med Assoc 2009, 234: 1559-1565.
49. Cockwill KR, Taylor SM, Snead EC, Dickinson R, Cosford K, Malek S, Lindsay LR, Diniz PP. Granulocytic anaplasmosis in three dogs from Saskatoon, Saskatchewan. Can Vet J 2009, 50: 835- 840.
50. Foley J, Drazenovich N, Leutenegger CM, Chomel BB. Association between polyarthritis and thrombocytopenia and increased prevalence of vectorborne pathogens in Californian dogs. Vet Rec 2007, 160: 159-162.
51. Kirtz G, Meli M, Leidinger E, Ludwig P, Thum D, Czettel B, Kolbl S, Lutz H. Anaplasma phagocytophilum infection in a dog: identifying the causative agent using PCR. J Small Anim Pract 2005, 46: 300-303.
52. Pusterla N, Huder J, Wolfensberger C, Litschi B, Parvis A, Lutz H. Granulocytic ehrlichiosis in two dogs in Switzerland. J Clin Microbiol 1997, 35: 2307-2309.
53. Chandrashekar R, Mainville CA, Beall MJ, O’Connor T, Eberts MD, Alleman AR, Gaunt SD, Breitschwerdt EB. Performance of a commercially available in-clinic ELISA for the detection of antibodies against Anaplasma phagocytophilum, Ehrlichia canis, and Borrelia burgdorferi and Dirofilaria immitis antigen in dogs. Am J Vet Res 2010, 71: 1443-1450.
54. Mylonakis ME, Koutinas AF, Theodorou K, Siarkou VI, Kontos VI. Clinical relevance of serologic testing in canine monocytic ehrlichiosis (Ehrlichia canis). Hellenic Vet Med Soc 2012, 63: 127- 134.
55. McCall JW, Baker CF, Mather TN, Chester ST, McCall SD, Irwin JP, Young SL, Cramer LG, Pollmeier MG. The ability of a topical novel combination of fipronil, amitraz and (S)-methoprene to protect dogs from Borrelia burgdorferi and Anaplasma phagocytophilum infections transmitted by Ixodes scapularis. Vet Parasitol 2011, 179: 335-342.
56. Bakken JS, Dumler JS. Clinical diagnosis and treatment of human granulocytotropic anaplasmosis. Ann N Y Acad Sci 2006, 1078: 236-247.
57. Chochlakis D, Psaroulaki A, Kokkini S, Kostanatis S, Arkalati E, Karagrannaki E, Tsiatis K, Tselentis Y, Gikas A. First evidence of Anaplasma infection in Crete, Greece. Report of six human cases. Clin Microbiol Infect 2009, 15 Suppl 2: 8-9.
58. Chochlakis D, Papaeustathiou A, Minadakis G, Psaroulaki A, Tselentis Y. A serosurvey of Anaplasma phagocytophilum in blood donors in Crete, Greece. Eur J Clin Microbiol Infect Dis 2008, 27: 473-475.



