Remember how...
Vaginal smear cytological examination of the bitch
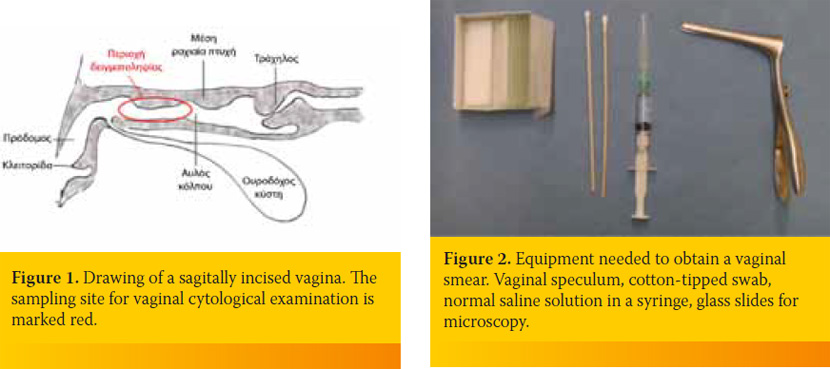
The vaginal smear cytological examination may be used to distinguish the stages of the ovarian cycle and to assist diagnosis of various genital system disorders. The vaginal cytological findings are evaluated always in combination with reproductive history data, genital system clinical findings (abdominal palpation, vaginoscopy, vaginal palpation) and when necessary, in combination with findings of genital system diagnostic imaging (ultrasonograpy and x-ray) and hormonal estimations.
Vaginal smear cytological examination allows the detection of the types and changes of superficial epithelial vaginal cells, which reflect the respective vaginal mucous membrane histological changes, induced by the ovarian cycle hormones. Especially oestrogens, that induce vaginal mucous membrane hyperplasia and hypertrophy, cause the most characteristic change in vaginal cytological picture i.e. the vaginal epithelial cell maturationhypertrophy and finally keratinization. This change starts during proestrus and peeks during oestrus, following the respective increase in proestrusoestrus oestrogens concentration with a delay of about four days to achieve cellular response. This change allows the detection of oestrus, which currently remains the most common indication for vaginal smear examination in lab-clinical practice.
TECHNIQUE OF VAGINAL SMEAR PREPARATION
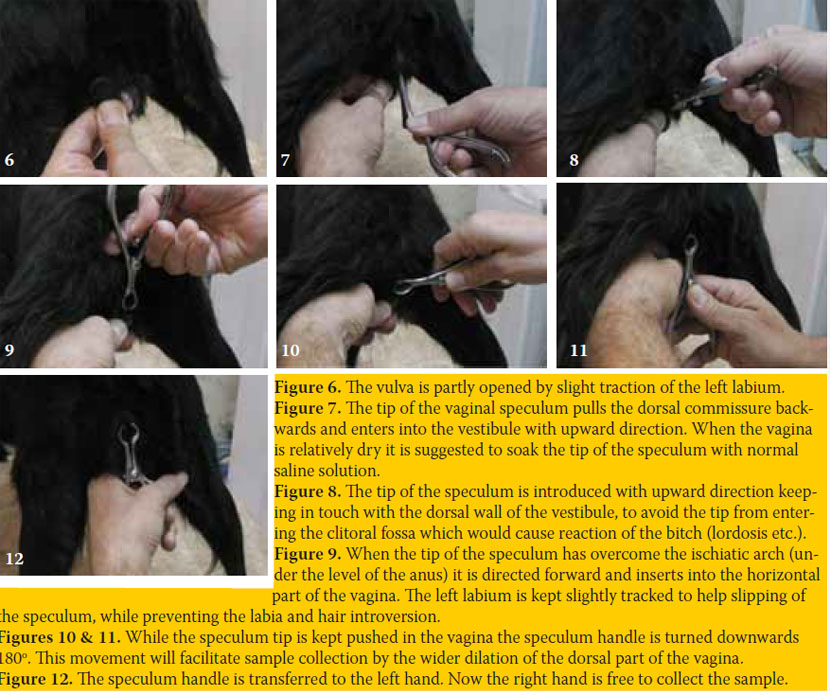
Obtaining the specimen
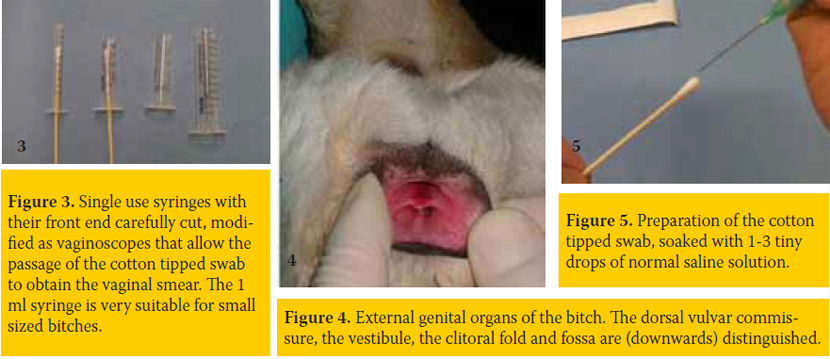
The best epithelial cell sampling site for the ovarian cycle staging is the dorsal, foremost-horizontal part of the vagina (figure 1). This site is better approached using a canine vaginal speculum (figure 2) or a suitable size canine cylindrical or other moment-modified vaginoscope (figure 3). Sampling from the vestibule, clitoral fossa or urethral orifice site should be avoided, because their cells are less representative of the endocrine pattern (figure 4). The sample is best obtained using a long cotton– tipped swab (14-15 cm), with the swab slightly moistened by 1 to 3 small drops of normal saline solution (figure 5). The passage of the speculum in the vagina and its dilation to obtain the specimen is depicted in figures 6-12.
The cotton-tipped swab is introduced through the speculum into the dilated vagina, carefully to avoid touching of the swab on the skin or hair (figures 13-16). The swab touches the mucous membrane and then mildly rotated while in touch to collect the specimen. The quantity of the collected cells and the thickness of the respective smear are always enough during proestrus and oestrus, while these are often scarce during dioestrus and anoestrus. Cell harvest may be increased with repeated washes of the vagina using a few drops of normal saline solution (1-3 ml). Vaginal discharge (normal or pathological) may be also collected from the bottom of the vagina, in a similar way, without rotation of the swab or using a plastic pipette.
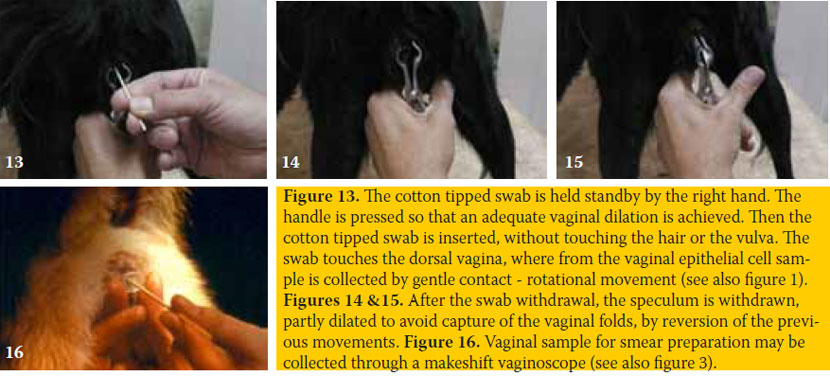
Attention is necessary to avoid vaginal trauma, either due to misdirection of the speculum or due to sudden movements of the bitch, often trying to avoid vaginal handling. Common reactions of the bitch during vaginal handling are the flexion of the loin, trying to sit on buttocks and raise standing only on rear limbs. Therefore it is advised to muzzle and restrain the dog to avoid accidents. It is quite safe if the handler keeps a hand under the inguinal – pelvic symphysis area to support the pelvis and immobilize the loin and backside during dilation. Yet, if the dog is strongly reacting, the speculum is immediately withdrawn, even without sample collection.
Fixation, staining and observation
After smear preparation, by rolling the swab on a clean glass slide (figure 17), it is allowed to air dry. Then it is fixed with methanol (covering or dipping of the slide). Methanol is also allowed to air dry for 15 minutes or less e.g. 5 minutes. Fixation is also possible using other fixatives indicated for specific staining methods.
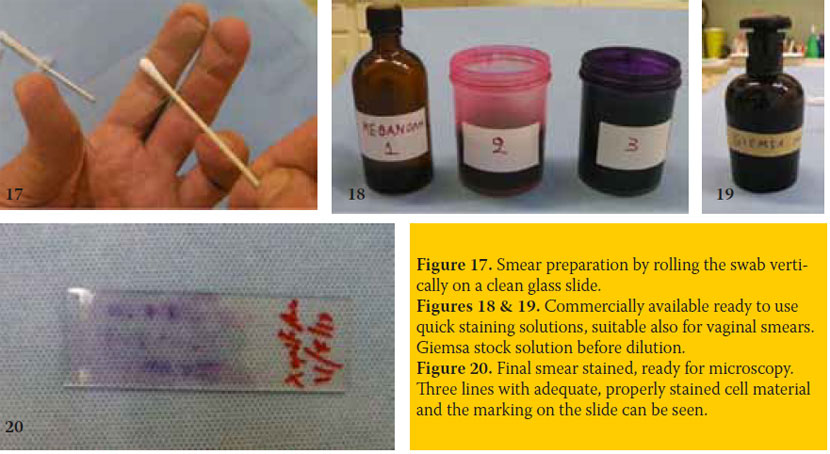
The fixed smear may be stained with Romanowski techniques, like Giemsa, Dip-Quick or Diff-Quik (and other similar quick staining techniques), Wright’s- Giemsa, Shorr’s, Papanicolaou etc. (figures 18 & 19). Intracellular details may become recognized with some of these stains. In practice, enough data for distinguishing the stages of the ovarian cycle is gained by recognizing mainly the size, the degree of maturity and the relative percentage of vaginal epithelial cells and secondarily that of neutrophils and erythrocytes. An easy method for the practitioner proves to be the Dip- Quick, which is accomplished in one minute, by three steps: 1st methanol fixation, 2nd and 3rd step: dipping of the glass slide for 5-8 seconds into the 2nd and 3rd staining solution (figure 18). Staining is directly followed by a gentle water rinse and a final air dry with the slide standing upright. Satisfactory and much cheaper is the Giemsa staining which is accomplished in about 20 minutes and includes methanol fixation, smear covering with Giemsa working solution (i.e. stock solution diluted v/v: 1/9 with distilled water or better with phosphate buffer at pH 6.8) for 10 to 20 minutes, time related to low or high smear cell content. Staining is directly followed by a gentle water rinse and a final air dry with the slide standing upright (Boon & Drijver 1986). At least the animal code or name and the day of sampling are permanently noted on one side of the glass slide (figure 20), which is necessary especially when series of samples are collected at different time points from the same animal.
The smear is examined with a simple microscope, using the x10 lens (x100 magnification) and for the detection of intracellular details using x40 or x100 oil lens. Many fields are studied (x100), first to distinguish the types of epithelial cells (size – maturity) that dominate in the smear and to roughly calculate their percentages %. Then the presence - abundance of other cell types, like erythrocytes and neutrophils is roughly quantified: absence (-), scarce (±) few (+), many (++) or too many (+++). These data allow the observer to distinguish the stages of the ovarian cycle. Further examination of intracellular details is sometimes indicated to support the diagnosis of probable genital system disorders.
MAIN VAGINAL EPITHELIAL CELL TYPES
The main types of vaginal epithelial cells that have been described (Olson et al. 1984, Boscos 2004) are:
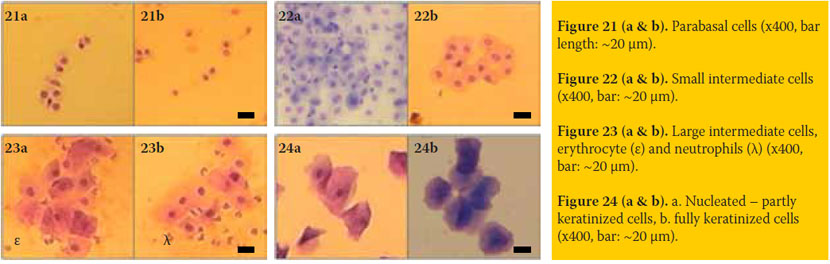
- Parabasal cells (figure 21): These are the smallest (mean diameter: 15μ, range: 9-20μ) and less mature vaginal epithelial cells observed. These have round or oval shape, normal nucleus (mean diameter: 10μ, range: 6-16μ) and highest nuclear-cytoplasmic ratio (mean: 0.7, range: 0.6-0.9), compared to the rest cell types.
- Small intermediate cells (figure 22): Their nucleus has the same size and shape, but their cell size is about double (m.d.: 30μ, range: 22-35μ), compared to that of the parabasal cells. These have lower nuclearcytoplasmic ratio (mean: 0.4, range: 0.3-0.5).
- Large intermediate cells (figure 23): These are large squamous cells (m.d.: 50μ, range 40-70μ) with irregular polyhedral shape and with distinct nucleus, of the same size to nuclei of the two previous cell types (mean nuclear-cytoplasmic ratio: 0.2).
- Keratinized (superficial squamous or cornified, figure 24): Their size and shape is similar to that of the large intermediate cells, but these have no nucleus (fully keratinized) or a pyknotic or barely discernible (0-8μ) nucleus (partly keratinized).
CYTOLOGICAL PATTERNS DURING THE STAGES OF THE OVARIAN CYCLE
The classic cytological patterns and alterations detected in the vaginal smears during the various stages of the ovarian cycle are described below:
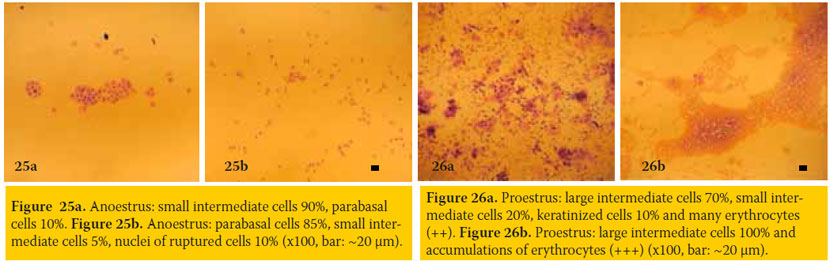
Anoestrus (figures 25a & 25b)
- Epithelial cells: Mainly parabasal and small intermediate cells, usually in small numbers and sometimes only nuclei of ruptured cells (without cytoplasm) are distinguished.
- Neutrophils: Absent or only in small numbers.
- Erythrocytes: Not present, unless minor trauma had occurred during sampling.
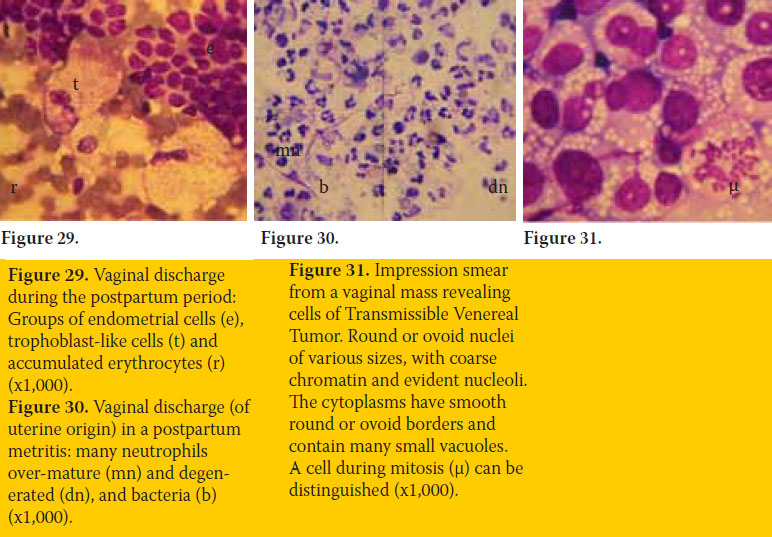
Comments about anoestrus: The smear is often sub-cellular and sometimes appears as if it was prepared by improper technique. Anoestrus also follows parturition, initially as postpartum period during which, uterine origin vaginal discharge and respective cell types may be detected (figure 29).
Proestrus (figures 26a & 26b)
- Epithelial cells: The numbers of parabasal and small intermediate cells progressively decrease, while the numbers of large intermediate and keratinized cells progressively increase.
- Neutrophils: Small numbers may be detected during early proestrus, while these progressively disappear during advanced proestrus.
- Erythrocytes: Usually present in high numbers during the whole proestrus.
Comments about proestrus: Sometimes erythrocytes are not detected, despite the obvious presence of sanguineous vaginal discharge. Some smears obtained during proestrus may look similar to those of dioestrus. Repetition of smear collection, every two days, is indicated in such doubtful cases to ascertain the stage of the ovarian cycle and its progress.
Oestrus (figures 27a & 27b)
- Epithelial cells: Their majority is keratinized (60-90% of total numbers) and the rest are large intermediate cells.
- Neutrophils: These are absent during oestrus. Sometimes few neutrophils appear during ending oestrus.
- Erythrocytes: These may be present in small or large numbers, or absent. These are not important.
Comments about oestrus: The cytological pattern of oestrus is characteristic and demonstrative of the general fertile period of the bitch. Although laborious, the repetition of copulations or inseminations every two days during the whole oestrus proves to be successive, when both parents have good fertility. Sometimes a more accurate detection of the 2-3 most fertile days of the bitch is necessary, by estimating progesterone concentration in serial daily measurements. The first important increase of progesterone concentration (>8-10 ng/ml of blood serum) indicates the beginning of ovulations and denotes the 2-4 days after that point as the most fertile, to program copulations or fresh semen inseminations. In successful pregnancy, parturition day may be predicted to occur at 63±1 days after ovulations. The percentage of keratinized to the total fo epithelial cells is often referred as the “eosinophilic index”.
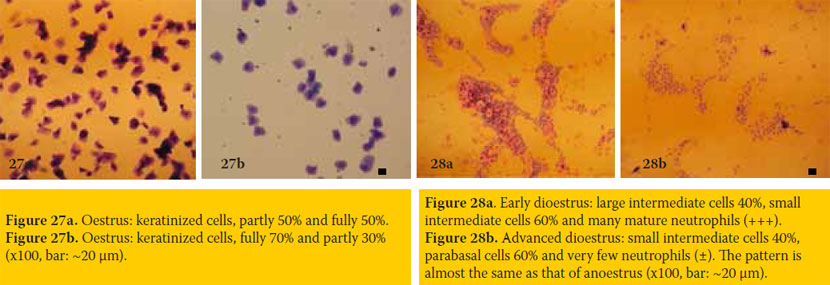
Dioestrus (figures 28a & 28b) or pregnancy
- Epithelial cells: Characteristic changes accompany the end of “cytological” oestrus and the beginning of “cytological” dioestrus (or metoestrus), occurring during a short period of 1-3 days. The keratinized cells disappear while parabasal and small intermediate cells reappear and dominate. During the rest of dioestrus, there is a progressive decrease in numbers and size of epithelial cells obtained, so that the cytological pattern becomes similar to that of anoestrus.
- Neutrophils: A sharp increase in the numbers of mature neutrophils is detected in the beginning of dioestrus. Subsequently, neutrophil numbers sharply decrease, so that small numbers of them are detected during the rest of dioestrus.
- Erythrocytes: These usually disappear, although small numbers may be detected sometimes during early dioestrus. These are not important.
Comments about dioestrus: The characteristic change of cytological pattern occurring at the end of oestrus – beginning of dioestrus allows the approximate prediction of the day of parturition (usually after 57±1 days). The cytological patterns of dioestrus and pregnancy are similar; although in some pregnancies large numbers of large intermediate cells are detected, without that being a secure criterion to differentiate dioestrus from pregnancy.
It is noteworthy, that changes in the cellular content of the vaginal smear are often detected during other normal conditions or disorders of the genital system. (Olson. 1984). Typical examples are the presence of a) endometrial cells and trophoblast-like cells during postpartum period (figure 29) or during sub-involution of placental sites, b) spermatozoa after copulation, c) large numbers of degenerated neutrophils (purulent discharge) in cases of open cervix pyometra, postpartum metritis (figure 30) or vaginitis, d) copious mucus in cases of vaginitis or advanced pregnancy, e) neoplasm round cells in case of Transmissible Venereal Tumor (figure 31) and f) the continuous presence of keratinized cells in cases of hyperoestogenism.
> References
1. P.N. Olson, M.A. Thrall, P.M. Wykes, T.M. Nett. “Vaginal Cytology. Part I. A Useful Tool for Staging the Canine Estrus Cycle” The Compendium on Continuing Education 6(4): 288-297, 1984
2. Μ.E. Boon & J.S. Drijver. Routine Cytological Staining Techniques. MACMILLAN, Hampshire 1986.
C. Boscos: Carnivore Reproduction Notes. A.U.Th., Thessaloniki, 2004 (in Greek).
4. P.N. Olson, M.A. Thrall, P.M. Wykes, T.M. Nett. “Vaginal Cytology. Part II. Its use in Diagnosing Canine Reproductive Disorders” The Compendium on Continuing Education 6(5): 385-390, 1984



