> Abstract
Dermatologic emergencies are rare in clinical practice and most commonly have an acute onset. A canine or feline skin disease can become life-threatening because of sepsis and toxemia, loss of fluids, protein, and electrolytes and the simultaneous involvement of vital internal organs. Emergency skin diseases include bacterial cellulitis in dogs, necrotizing fasciitis in dogs and cats, toxic shock syndrome in dogs, subcutaneous and systemic fungal infections in dogs and cats, angioedema in dogs and cats, autoimmune skin diseases with extensive ulceration in dogs and cats, Stevens-Johnson syndrome and toxic epidermal necrolysis in dogs and cats, vasculitis in dogs and cats, sterile postural erythroderma (superficial suppurative necrolytic dermatitis) of miniature Schnauzers, sulfonamide hypersensitivity syndrome in dogs, and sterile neutrophilic dermatosis (subcorneal and follicular neutrophilic pustular dermatitis or Sweet’s syndrome) in dogs. The diagnosis of these diseases is based on history, clinical signs and the results of various laboratory examinations (cytologic, microbiologic, histopathologic etc.). However, in addition to the definitive diagnosis of the skin disease itself, clinical and laboratory (complete blood count, serum biochemistry, urinalysis, coagulation profile etc.) examinations for potential systemic complications are also important. Treatment should begin as soon as possible and includes the general supportive measures applicable to all emergency cases and the specific treatment of the skin disease.
> Introduction
Most canine and feline skin diseases, irrespectively of their acute or progressive onset and of their influence on the animal’s and the owner’s quality of life,1-3 are not life-threatening. This permits a gradual diagnostic approach, starting with the most necessary, least time-consuming and less expensive examinations, gives a time window to perform therapeutic trials, when they are indicated, and allows treatment on an outpatient basis.4 On the contrary, dermatologic emergencies, that are not common in the clinical practice, are life-threatening and consequently necessitate fast diagnosis, hospitalization and aggressive treatment in order to increase the chances for a favorable outcome.5-7
Most dermatologic emergencies have a sudden onset with the exception of some chronic skin diseases, usually of infectious etiology or with extensive ulceration of the skin, where proper treatment had not been applied. The basic pathomechanisms that can threaten the animal’s life are sepsis and toxemia, considerable loss of fluids, protein and electrolytes and simultaneous involvement of vital internal organs (Table 1).8
The aim of this article is to review the etiology, pathogenesis, clinical signs, diagnosis and emergency treatment of the main dermatologic emergencies of dogs and cats. It is emphasized that only the therapeutic interventions that target the skin disease are reported, whereas treatment of sepsis, toxemia, dehydration, hypoproteinemia, electrolyte imbalances, internal organ dysfunction and of the ensuing syndromes (systemic inflammatory response syndrome, multi-organ dysfunction syndrome, disseminated intravascular coagulation etc.) are beyond the scope of this review.
> Bacterial cellulitis in dogs
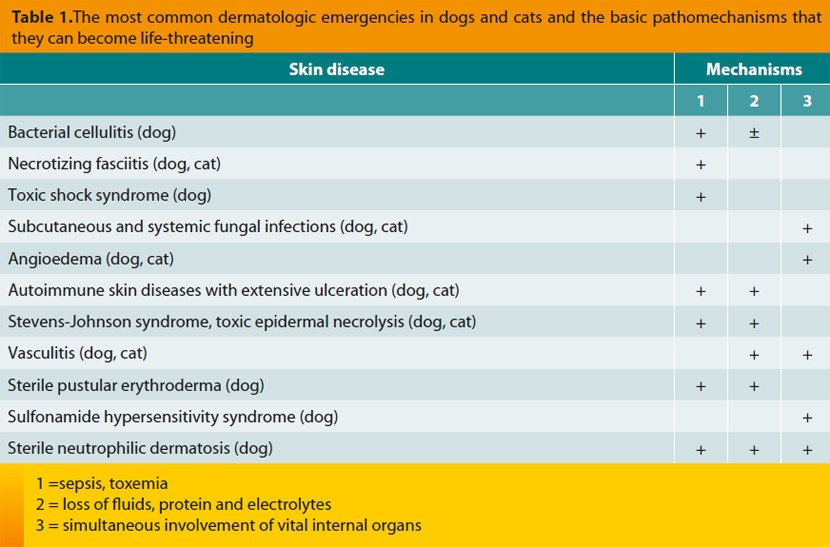
Bacterial cellulitis is a septic inflammation of the subcutaneous fat resulting from infection by common microorganisms. When it is multifocal or generalized, it can become life-threatening, mainly due to toxemia and sepsis. In most cases, skin lesions begin as superficial folliculitis, evolve in deep folliculitis-furunculosis and eventually result in cellulitis. The most common microorganisms that are isolated from such lesions include Staphylococcus pseudintermedius, Pseudomonas aeruginosa, Escherichia coli, Proteus sp. and anaerobic bacteria (e.g. Clostridium sp., Bacteroides sp.) and mixed infections are not uncommon.9 Potential underlying causes are multiple and essentially identical with those which predispose to superficial folliculitis and to deep folliculitis-furunculosis. However, in most dogs where the skin infection evolves in multifocal or generalized cellulitis with sepsis or toxemia, the primary cause is the generalized demodicosis due to Demodex canis (Figure 1) or the compromised immune responses of the skin, for example after the systemic administration of glucocorticoids for a prolonged time period or in immunosuppressive dosage regimens or in the case of deep folliculitis, furunculosis and panniculitis of German Shepherds.10
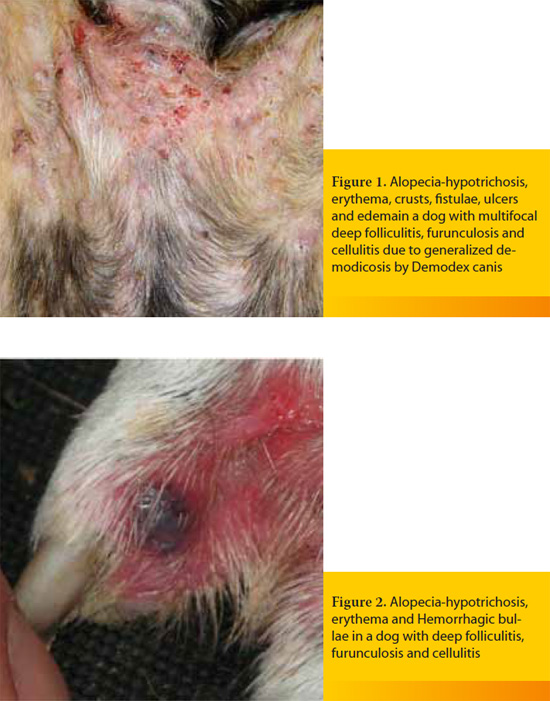
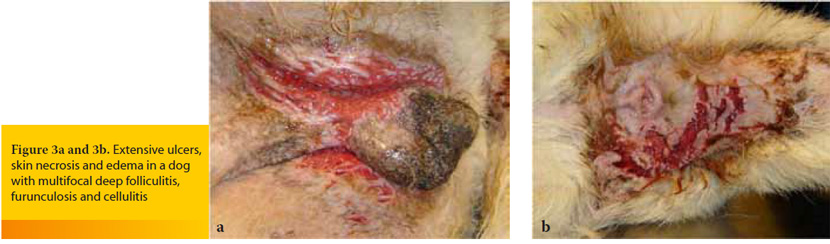
Macroscopic skin lesions are identical with those of deep folliculitis-furunculosis, and they are characterized by nodules, hemorrhagic bullae (Figure 2), furuncles, fistulae extruding pyohemorrhagic exudate that forms hemorrhagic crusts, ulcers and edema (Figure 3).9-11 Diagnosis is relatively straightforward and it is based on the clinical signs and the results of cytological examination after superficial cleaning of the lesions, that typically reveals pyogranulomatous inflammation with degenerate neutrophils, activated macrophages and relatively few microorganisms.9,11 Although cutaneous histopathology can confirm the spread of the infection into the subcutaneous fat, it is not necessary for initial diagnosis and treatment. However, it can be considered, after the initial stabilization of the patient, when the skin lesions do not show a satisfactory response to the properly selected antimicrobial treatment. In such cases, suspicion arises that cellulitis by common microorganisms may be secondary to another infection by specific pathogens, like mycobacteria or those fungal species that cause subcutaneous and systemic mycoses. In contrast to histopathology, culture and in vitro antimicrobial susceptibility testing, as well as all the necessary laboratory examinations to investigate for the underlying cause are mandatory for the selection of the optimal antimicrobial treatment and for the definitive management of bacterial cellulitis, respectivelly. 9
Initial treatment includes the stabilization of the dog (treatment of the systemic inflammatory response and the multi-organ dysfunction syndromes), the topical therapy (hair clipping, hydrotherapy, antiseptic baths) and the parenteral administration of antimicrobials.9 The latter must be selected based on the results of in vitro antimicrobial susceptibility testing. However, until the results become available (usually after 48 hours), empirical treatment with a combination of antimicrobials that is expected to be effective against as many of the responsible microorganisms as possible is necessary. For the first critical 48 hours, the author usually selects a combination of amoxicillin- clavulanic acid (20mg/kg of body weight, intravenously, every 8 hours), clindamycin (11mg/ kg of body weight, intravenously, every 12 hours) and enrofloxacin (20mg/kg of body weight, subcutaneously or slowly intravenously after dilution in a large volume of normal saline, every 24 hours). If there is no indication of renal dysfunction, enrofloxacin can be replaced by amikacin (15-30mg/kg of body weight, subcutaneously, intramuscularly or intravenously, every 24 hours).9
> Necrotizing fasciitis in dogs and cats
This rare infection usually occurs following direct inoculation of the responsible microorganisms into the subcutaneous tissue, for example after minor skin trauma or after intravenous catheterization when aseptic conditions are not met.12 Usually only one bacterial species is isolated (type ΙΙ necrotizing fasciitis by Streptococcus canis or S. pseudintermedius and less commonly by Acinetobacter baumannii, E. coli or Prevotella bivia), although mixed infections (type I necrotizing fasciitis) have also been reported.12-17 Skin lesions are the consequence of the bacterial toxins, of the severe edema that causes local ischemia, and perhaps of the vasculitis.11,15
History and physical examination reveals edema of sudden onset that initially involves one area of the body but subsequently spreads and is accompanied by severe pain.12,16,17 Later on, the skin becomes cyanotic with vesicles and bullae and eventually necrosis, ulceration and crusting appears. 13,15, 16 Definitive diagnosis is based on cytology (suppurative or pyogranulomatous septic inflammation), on culture and in vitro antimicrobial susceptibility testing (isolation of the offending microorganism) and on cutaneous ultrasonography (demarcated pockets with fluid accumulation in the subcutaneous tissue).13,16,17
Treatment is similar to that of bacterial cellulitis with the addition of immediate surgical intervention for removal of necrotic tissues and for drainage of the exudate.12,16,17 Based on a limited number of published cases,13,14 the prognosis for affected dogs and cats appears to be guarded to poor, like the situation in humans, in whom the mortality rate may be as high as 70% when patients are admitted with signs of sepsis.7
> Toxic shock syndrome in dogs
In humans, toxic shock syndrome usually appears after overgrowth or infection by Staphylococcus aureus that produces toxins, like toxic shock syndrome toxin-1 (TSST-1) and enterotoxins. These toxins act as superantigens that stimulate the massive release of cytokines, such asinterleukin-1 (IL-1) and tumor necrosis factor-a (TNF-a) by Tlymphocytes. These cytokines are considered responsible for both the skin lesions (generalized erythematous macules) and the systemic signs (fever, hypotension, multi-organ dysfunction syndrome). In most of the cases the primary infection does not involve the skin and the nidus of the infection may not clinically evident.18,19
A similar syndrome has been reported in a small number of dogs. In most of the cases the site of the infection remained obscure, the skin lesions were characterized by generalized erythematous macules and edematous plaques, the systemic signs included fever and anorexia, and the laboratory findings were indicative of the syndrome of disseminated intravascular coagulation (DIC).11,20,21 Unfortunately, the diagnosis of toxic shock syndrome was not definitively proved in these dogs by measuring the concentration of staphylococcal toxins. However, this disease entity is probably analogous to the human toxic shock syndrome, considering the clinical, laboratory and histopathologic similarities along with the fact that S. pseudintermedius can produce toxins that are similar to those of S. aureus, such as enterotoxin C.11,20,22,23
Treatment includes the stabilization of the patient, the management of DIC and the parenteral administration of antimicrobials, like cephalexin or fluoroquinolones.20 Prognosis is guarded to poor, considering the 50% mortality rate,11,21 but it may become more favorable if the nidus of infection is found and surgically treated.20
> Subcutaneous and systemic fungal infections in dogs and cats
Certain fungal organisms that cause skin lesions may simultaneously affect internal organs, including central nervous system. In such cases, the skin lesions will be accompanied by neurologic signs and the disease may become life-threatening. Responsible organisms include Paecilomyces sp., fungi that cause phaeohyphomycosis and mucormycosis, Sporothrix schenkii (mainly in cats), Blastomyces dermatitidis, Coccidioides immitis (in dogs), Cryptococcus sp., Histoplasma capsulatum and rarely Prototheca zopfii (in dogs).10,11,24 Although the fungal infections do not appear to be particularly common in Greece, a case of lethal generalized feline cryptococcosis has been reported. In that case, besides skin lesions, involvement of almost every internal organ, including the central nervous system, was observed in postmortem examination.25
The identification of the fungal cause of skin lesions with the aid of cytology, culture (it should be performed only in specialized laboratories when there is suspicion of blastomycosis, coccidioidomycosis, orhistoplasmosis, due to the high risk of infection for the laboratory personnel), histopathology and perhaps other more specialized examinations, will guide the diagnostic investigation for internal organ infection and will permit immediate implementation of appropriate treatment. 10,11 The latter includes supportive measures, symptomatic treatment, and the systemic administration of antifungals reaching therapeutic concentrations in the central nervous system, like fluconazole, liposomal amphotericin B and flucytosine.10,24
> Angioedema in dogs and cats
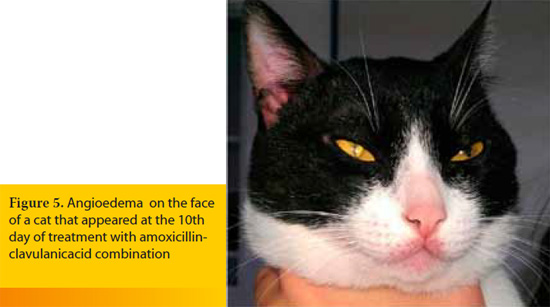
Angioedema is caused by extensive degranulation of cutaneous mast cells that release massive amounts of inflammatory mediators, including histamine. The end result is diffuse edema of the dermis and the subcutaneous tissue.11,26 It usually represents a type I hypersensitivity reaction with many possible underlying causes, such as drugs, vaccines (usually containing inactivated organisms, like those against canine leptospirosis), blood transfusion and insect bites (Figures 4 and 5).11,26,27 Angioedema can become fatal if it expands into the soft tissues of the pharynx and larynx leading to obstruction of the upper respiratory tract or if it progresses to anaphylactic shock.10,27 In the latter case, degranulation of blood basophils may contribute to the pathogenesis.

Skin lesions appear abruptly, they usually involve the head, they can be pruritic and they may be accompanied by symptoms of upper airway obstruction (inspiratory dyspnea with stridor) and/or of anaphylactic shock.10,11,26 Diagnosis is based on history and clinical signs. Immediate therapeutic measures include: a) treatment for airway obstruction and/or anaphylactic shock, b) cold compresses, c) administration of epinephrine at the dose of 0.01-0.02mg/kg of body weight intramuscularly, subcutaneously or intravenously (the commercial preparation is diluted 1:10 with normal saline and 0.1-0.2ml/kg of body weight of the diluted solution is administered), d) administration of antihistamines, like diphenhydramine at the dose of 0.5-2mg/kg of body weight intramuscularly or slowly intravenously. Antihistamines prevent further activation of the H1 receptors by histamine and thus the ensuing vasodilation and increased vascular permeability, e) perhaps the administration of fast-acting glucocorticoids, like dexamethasone (1-2mg/kg of body weight, intravenously) or methylprednisolone sodium succinate (10-25mg/kg of body weight, intravenously).10, 27
> Autoimmune skin diseases with extensive ulceration in dogs and cats
These are rare diseases characterized by loss of skin adhesion, localized in the deeper layers of the epidermis (pemphigus vulgaris and paraneoplastic pemphigus) or in the dermo-epidermal junction (e.g. epidermolysis bullosa acquisita, vesicular cutaneous lupus erythematosus) (Figure 6).11,27-29 Frequently skin lesions are accompanied by ulceration of the oral mucosa.11,27,29 The evolution is usually progressive, although there are cases that are admitted as emergencies, mostly due to secondary bacterial infections that may lead to sepsis and due to loss of fluids, protein and electrolytes from the widespread skin lesions.11,30
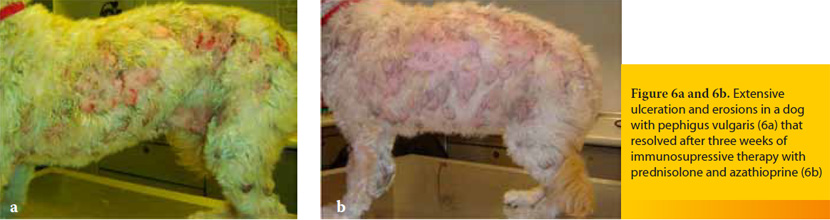
Diagnosis relies on histopathology and perhaps on additional more specialized laboratory examinations and long-term treatment includes the administration of immunosuppressive drugs, such as prednisolone, azathioprine (dogs only) and chlorambucil.29-31 On the contrary, the initial management in case of systemic signs is similar to that of bacterial cellulitis with the addition of the topical application of silver sulfadiazine ointment and the aseptic bandaging of the ulcers.27
> Stevens-Johnson syndrome and toxic epidermal necrolysis in dogs and cats
Currently, human Stevens-Johnson syndrome and toxic epidermal necrolysis are considered to be a single disease entity that differs from erythema multiforme in terms of its epidemiology, etiopathogenesis, clinical signs, histopathologic findings, response to treatment and prognosis.32 In veterinary dermatology there is still controversy because some authors support the idea that Stevens-Johnson syndrome and toxic epidermal necrolysis represent stages or a more severe clinical phenotype of erythema multiforme. However, etiopathogenesis, clinical signs, and prognosis do not corroborate this point of view.11,33,34
Stevens-Johnson syndrome and toxic epidermal necrolysis are characterized by massive necrosis or apoptosis of epidermal and hair follicle keratinocytes.11,26 In humans, the underlying pathomechanisms are complex and include the interaction between the Fasmolecule and its ligand (FasL) on the surface of keratinocytes, the release of cytokines, the interaction between antigen-presenting cells and T-lymphocytes and the action of cytotoxic lymphocytes.11,26,32 The most common underlying cause, in both humans (75% of the cases) and dogs (92% of the cases) is the administration of drugs, including various antibacterial, antifungal and antiparasitic agents, immunomodulatory and antiepileptic drugs etc. 6,7,26,32,33,35,36 Particularly in humans, there is a lag period of 4 days up to 4 weeks between the first administration of the offending medication and the appearance of the skin lesions and the systemic signs.6 Other less common causes of Stevens-Johnson syndrome and toxic epidermal necrolysis include infections and neoplasia, where as idiopathic cases also exist.11,32,33
In dogs and cats, the lesions appear suddenly, they are mainly characterized by vesicles and bullae that rapidly progress to ulcers and by extensive epithelial necrosis and they involve the skin, the claws, the mucocutaneous junctions and the mucosae.11,33,35,36 At the same time, systemic signs that are caused mainly by sepsis and loss of fluids, protein and electrolytes, are noted.26,33,35,36 In humans, mortality rate is high and varies from 10% in Stevens-Johnson syndrome up to 50% in toxic epidermal necrolysis, because epidermal necrosis involves less than10% and more than 30% of the body surface area, respectively.6,7,26,32 Based on a limited body of scientific information, it appears that the mortality rate in dogs with toxic epidermal necrolysis approaches 100%.26,33 Although histopathologic examination is the only way to definitively confirm the diagnosis, the rapid progression of this disease necessitates imminent implementation of therapeutic measures.33 The latter include the discontinuation of the offending drug, the avoidance of drugs with similar chemical structure, and the supportive treatment (administration of fluids, electrolytes and colloids, whole blood or plasma transfusion, systemic and topical antimicrobials, bandaging the lesions, analgesia etc.).32,35 In addition, immunosuppressive doses of glucocorticoids, intravenous human immunoglobulin, cyclosporine A and pentoxifylline have been tried in some patients.31 Currently, in both human and veterinary medicine, there is insufficient evidence to support the effectiveness of these treatment options. Glucocorticoids may be of some value but they can also become detrimental, especially in patients with sepsis.36 Intravenous human immunoglobulin (single administration at the dose of 0.5-1.5g/kg of body weight, slowly over 6-12 hours) appears to be effective, at least in certain patients, but it is expensive and it may cause adverse effects, mostly anaphylaxis.36,37 Its basic mechanism of action in dogs and cats with Stevens-Johnson syndrome and toxic epidermal necrolysis appears to be the inhibition of the binding of Fas molecule to its ligand.31,36,37
> Vasculitis in dogs and cats
The term vasculitis describes the inflammation of the blood vessel wall, whereas vasculopathy signifies the result of vascular insufficiency that is not associated with active inflammation of the vessels.11,29 However, vasculopathy may be an evolutionary stage of vasculitis and is diagnosed most commonly when the biopsy samples for histopathologic examination are obtained long after the appearance of skin lesions.11 The pathomechanisms that are responsible for the development of vasculitis are usually immune-mediated and in most cases a type III hypersensitivity reaction is implicated.11,26,27 The underlying causes vary (e.g. infections, exposure to toxins, drugs, vaccines, food allergy, neoplasia, various systemic diseases) and idiopathic cases are not rare.11, 26, 27, 29 When canine and feline vasculitis is restricted to the skin, it is not typically life-threatening due to the relatively limited area of necrosis and ulceration (Figure 7). On the other hand, cutaneous vasculitis may require hospitalization and emergency treatment when: a) it is secondary to another systemic disease which is life-threatening, like bacterial endocarditis (Figure 8),11 b) it is associated with involvement of internal organs such as the kidney, the liver, the gastrointestinal tract, and the thoracic or abdominal serosal surfaces or with hemostatic disorders (e.g. thrombocytopenia, DIC), like in the syndrome of cutaneous and renal glomerular vasculopathy that is encountered most commonly, but not exclusively, in Greyhounds,11, 26, 38, 39 or c) it is causing acute and severe bleeding, like in hyperplastic arteritis of the nasal philtrum.11,38,40
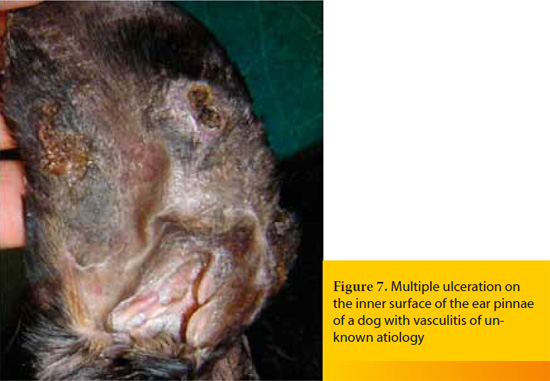
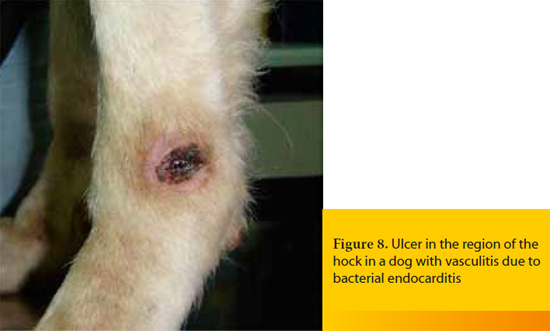
Skin lesions are variable (alopecia, erythema, hemorrhagic macules or purpura, edema, ulcers that are covered by hemorrhagic crusts), they can be painful and they usually appear on the extremities of the body where lateral circulation is limited, like the pinnae, the nasal philtrum, the paw pads, the bony prominences and the tip of the tail.26 Diagnosis is based on the combination of clinical signs and results of histopathology and it is not always straightforward because the macroscopic and the microscopic lesions vary depending on the underlying cause, the pathogenesis, and the chronicity of the disease.11, 27 In emergency cases measurement of fibrinogen and d-dimers concentration in the blood is particularly helpful, because the former is increased in almost all cases of cutaneous vasculitis and the latter when vasculitis is associated with thrombosis.38, 41 Furthermore, in every case, an extensive diagnostic investigation for the possible underlying causes and for the systemic complications of the disease, is mandatory27,29 and it should include, among others, bacterial and fungal cultures of aseptically obtained skin biopsy samples.38 The aims of the emergency treatment are the restoration of the function of the affected vital organs, the management of hemostatic disorders and the treatment of the underlying cause. In idiopathic cases, except than the usual immune modulating drugs (glucocorticoids, azathioprine in dogs, chlorambucil, gold salts), pentoxifylline, sulfasalazine, dapsone, cyclosporine A, tetracycline-niacinamide combination, and vitamin E may also be administered.27,29,31,38,40
> Sterile pustular erythroderma or superficial suppurative necrolytic dermatitis of miniature Schnauzers
This skin disease that bears many clinical and histopathologic similarities with sterile neutrophilic dermatosis (see below) has been reported exclusively in adult miniature Schnauzers.11,26,42 In most cases, skin lesions and systemic signs appear approximately 2-3 days after bathing with various medicated or nonmedicated shampoos.11,26,42 Pathogenesis remains obscure and it is considered to be an idiosyncratic drug eruption with genetic predisposition that is probably associated with mass release of cytokines by keratinocytes.
Skin lesions may be localized at the ventral aspects of the body trunk or may be generalized, and they are characterized by erythema, plaques, papules, vesicles and pustules that rapidly evolve into extensive ulceration and skin necrosis.11,42 Most patients present systemic signs, like fever and depression, and the laboratory investigation may reveal neutrophilic leucocytosis, hypoalbuminemia and findings indicative of DIC.11,26,42 Treatment is purely symptomatic and in those dogs that survive skin lesions self-heal in a period of 1-2 weeks.
> Sulfonamide hypersensitivity syndrome in dogs
It is a multisystemic syndrome that appears in a few dogs following the administration of sulfonamides, usually in combination with trimethoprim or ormetroprim.43 The underlying pathogenesis has not been fully elucidated, although the activation of cellular and humoral immunity against complexes formed by the drug or its metabolites and certain proteins of the body has been implicated.44 The most common systemic signs, syndromes or pathologic conditions noted in affected dogs are fever, liver disease, hematologic disorders (hemolytic anemia, leukopenia, eosinophilia, thrombocytopenia), renal disease, polyarthritis and uveitis.43
Skin lesions are noted in a minority of affected dogs and they usually appear in the form of maculopapular eruption or of exfoliative erythroderma.43 Diagnosis is based on history (the offending drug has been administered for 5-36 days before the appearance of clinical signs), the breed (Doberman pinchers, miniature Schnauzers and Samoyeds are over-represented), clinical signs and laboratory findings. Treatment is symptomatic43 and the mortality rate is 20% on average.43
> Sterile neutrophilic dermatosis or subcorneal and follicular neutrophilic pustular dermatitis or Sweet’s syndrome in dogs
It is a rare and probably immune-mediated skin disease of the dog which may be associated with various drugs and particularly with non-steroidal anti-inflammatories, like carprofen.11, 45 Systemic signs and probable death of the affected animals are attributed to the extensive ulceration of the skin, to the simultaneous involvement of internal organs and to the hematologic abnormalities (hemolytic anemia, immune-mediated thrombocytopenia) that commonly coexist.45
Skin lesions develop suddenly, they are often generalized, they may involve the mucocutaneous junctions, and they are characterized by erythema, pustules, target lesions (similar to those of erythema multiforme), crusts, erosions and ulcers.11,45,46 Definitive diagnosis is based on histopathologic examination, in which, other than neutrophilic dermatitis, vasculitis may also be noticed.45,46 Treatment includes supportive measures and administration of glucocorticoids at immunosuppressive dosage regimen, possibly in combination with azathioprine.45
> References
1. Favrot C, Linek M, Mueller R, Zini E, International Task Force on Canine Atopic Dermatitis. Development of a questionnaire to assess the impact of atopic dermatitis on health-related quality of life of affected dogs and their owners. Vet Dermatol 2010, 21: 64-70.
2. Linek M, Favrot C. Impact of canine atopic dermatitis on the health-related quality of life of affected dogs and quality of life of their owners. Vet Dermatol 2010, 21: 456-462.
3. Noli C, Colombo S, Cornegliani L, Ghibaudo G, Persico P, Vercelli A, Galzerano M.Quality of life of dogs with skin disease and their owners. Part 2: administation of a questionnaire in various skin diseases and correlation to efficacy of therapy. Vet Dermatol 2011, 22: 344-351.
4. Bergvall K. History, examination and initial evaluation. In: BSAVA Manual of Canine and Feline Dermatology. Jackson H,Marsella R (eds). 3rd edn. BSAVA: Gloucester, 2012, pp. 12-23.
5. Brady WJ, DeBehnke D, Crosby DL. Dermatological emergencies. Am JEmerg Med 1994, 12: 217-237.
6. Freiman A, Borsuk D, Sasseville D. Dermatologic emergencies. Can Med Assoc J 2005, 173: 1317-1319.
7. Usatine RP, Sandy N. Dermatologic emergencies. Am Fam Phys 2010, 82: 773-780.
8. Saridomichelakis MN. An approach to erosions and ulcerations. In: BSAVA Manual of Canine and Feline Dermatology. Jackson H,Marsella R (eds). 3rd edn. BSAVA: Gloucester, 2012, pp. 57-64.
9. Noli C, Morris DO. Staphylococcal pyoderma. In: BSAVA Manual of Canine and Feline Dermatology. Jackson H,Marsella R (eds). 3rd edn. BSAVA: Gloucester, 2012, pp. 173-187.
10. Paterson S. Manual of Skin Diseases of the Dog and Cat. 2nd edn. Blackwell Publishing,Oxford, 2008.
11. Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Skin Diseases of the Dog and Cat. Clinical and Histopathologic Diagnosis. 2nd edn. Blackwell Publishing,Oxford, 2005.
12. Worth AJ, Marshall N, Thompson KG. Necrotising fasciitis associated with Escherichia coli in a dog. N ZealVet J 2005, 53: 257-260.
13. Sura R, Hinckley LS, Risatti GR, Smyth JA. Fatal necrotising fasciitis and myositis in a cat associated with Streptococcus canis. Vet Rec 2008,162: 450-453.
14. Weese JS, Poma R, James F, Buenviaje G, Foster R, Slavic D. Staphylococcus pseudintermedius necrotizing fasciitis in a dog. Can Vet J 2009, 50: 655-656.
15. Brachelente C, Wiener D, Malik Y, Huessy D. A case of necrotizing fasciitis with septic shock in a cat caused by Acinetobacter baumannii. Vet Dermatol 2007, 18: 432-438.
16. Hess MO. Necrotising fasciitis due to Prevotella bivia in a cat. J Small Anim Pract 2009, 50: 558-560.
17. Csiszer AB, Towle HA, Daly CM. Successful treatment of necrotizing fasciitis in the hind limb of a great dane. J Am Anim Hosp Assoc 2010, 46: 433-438.
18. Feldman SR. Bullous dermatoses associated with systemic disease. Dermatol Clin 1993, 11: 597-609.
19. McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Ann Rev Microbiol 2001, 55: 77-104.
20. Declercq J. Suspected toxic shock-like syndrome in a dog with closed-cervix pyometra. Vet Dermatol 2007, 18: 41-44.
21. Slovak JE, Parker VJ, Deitz KL. Toxic shock syndrome in two dogs. J Am Anim Hosp Assoc 2012, 48: 434-438.
22. Yoon JW, Lee GJ, Lee SY, Park C, Yoo JH, Park HM. Prevalence of genes for enterotoxins, toxic shock syndrome toxin 1 and exfoliative toxin among clinical isolates of Staphylococcus pseudintermedius from canine origin. Vet Dermatol2010, 21: 484-489.
23. Hendricks A, Schuberth HJ, Schueler K, Lloyd DH. Frequency of superantigen-producing Staphylococcus intermedius isolates from canine pyoderma and proliferation-inducing potential of superantigens in dogs. Res Vet Sci2002, 73: 273-277.
24. Friberg C. Subcutaneous and deep infections. In: BSAVA Manual of Canine and Feline Dermatology. Jackson H,Marsella R (eds). 3rd edn. BSAVA: Gloucester, 2012, pp. 215-226.
Saridomichelakis MN, Mylonakis M, Koutinas AF, Kaldrymidou E, Fotiadou P. A case of generalized cryptococcosis in a cat. In: Proceedings of 6thPanhellenicConference ofSmall Animal Veterinary Medicine. Athens, 2002, p.134-140.
26. Voie KL, Campbell KL, Lavergne SN. Drug hypersensitivity reactions targeting the skin in dogs and cats. J Vet Intern Med 2012, 26: 863-874.
27. Medleau L, Hnilica KA. Small Animal Dermatology-A Color Atlas and Therapeutic Guide. 2nd edn. Saunders Elsevier,St. Louis, 2006.
28. Jackson HA, Olivry T. Ulcerative dermatosis of the Shetland sheepdog and rough collie dog may represent a novel vesicular variant of cutaneous lupus erythematosus. Vet Dermatol 2001, 12: 19-27.
29. Jackson HA. Autoimmune and immune-mdiated skin disease. In: BSAVA Manual of Canine and Feline Dermatology. Jackson H,Marsella R (eds). 3rd edn. BSAVA: Gloucester, 2012, pp. 206-214.
30. Outerbridge CA. Cutaneous manifestations of internal diseases. Vet Clin North Am-Small Anim Pract 2013, 43: 135-152.
31. Whitley NT, Day MJ. Immunomodulatory drugs and their application to the management of canine immunemediated disease. J Small Anim Pract 2011, 52: 70-85.
32. Mockenhaupt M. The current understanding of Stevens-Johnson syndrome and toxic epidermal necrolysis. Exp RevClin Immunol 2011, 7: 803-813.
33. Hinn AC, Olivry T, Luther PB, Cannon AG, Yager JA. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in the dog: clinical classification, drug exposure and histopathological correlations. JVet Allergy Clin Immunol 1998, 6: 13-20.
34. ΠαϊτάκηΧ, ΚουτίναςΑΦ, ΣαριδομιχελάκηςΜΝ. Το πολύμορφο ερύθημα στο σκύλο. Δελτίο της Ελληνικής Κτηνιατρικής Εταιρείας 2001, 52: 220-224.
35. Lee JA, Budgin JB, Mauldin EA. Acute necrotizing dermatitis and septicemia after application of a d-limonene-based insecticidal shampoo in a cat. J Am Vet Med Assoc 2002, 221: 258-262.
36. Nuttall TJ, Malham T. Successful intravenous human immunoglobulin treatment of drug-induced Stevens- Johnson syndrome in a dog. J Small Anim Pract 2004, 45: 357-361.
37. Trotman TK, Phillips H, Fordyce H, King LG, Morris DO, Giger U. Treatment of severe adverse cutaneous drug reactions with human intravenous immunoglobulin in two dogs. J Am AnimHosp Assoc 2006, 42: 312-320.
38. Innerå M. Cutaneous vasculitis in small animals. Vet Clin North Am-Small Anim Pract 2013, 43: 113-134.
39. Rotermund A, Peters M, Hewicker-Trautwein M, Nolte I. Cutaneous and renal glomerular vasculopathy in a great dane resembling ‘Alabama rot’ of greyhounds. Vet Rec 2002, 151: 510-512.
40. Torres SM, Brien TO, Scott DW. Dermal arteritis of the nasal philtrum in a Giant Schnauzer and three Saint Bernard dogs. Vet Dermatol 2002,13: 275-281.
41. Rosser EJJ. Use of the D-dimer assay for diagnosing thrombosis in cases of canine cutaneous vasculitis. Vet Dermatol 2009, 20: 586-590.
42. Murayama N, Midorikawa K, Nagata M. A case of superficial suppurative necrolytic dermatitis of miniature schnauzers with identification of a causative agent using patch testing. Vet Dermatol 2008, 19: 395- 339.
43. Trepanier LA, Danhof R, Toll J, Watrous D. Clinical findings in 40 dogs with hypersensitivity associated with administration of potentiated sulfonamides. J Vet Intern Med 2003, 17: 647-652.
44. Lavergne SN, Danhof RS, Volkman EM, Trepanier LA. Association of drug-serum protein adducts and anti-drug antibodies in dogs with sulphonamide hypersensitivity: a naturally occurring model of idiosyncratic drug toxicity. Clin Exp Allergy 2006, 36: 907-915.
45. Mellor PJ, Roulois AJ, Day MJ, Blacklaws BA, Knivett SJ, Herrtage ME. Neutrophilic dermatitis and immunemediated haematological disorders in a dog: suspected adverse reaction to carprofen. J Small Anim Pract 2005, 46: 237-242.
46. Johnson CS, May ER, Myers RK, Hostetter JM. Extracutaneous neutrophilic inflammation in a dog with lesions resembling Sweet’s Syndrome. Vet Dermatol 2009, 20: 200-205.



